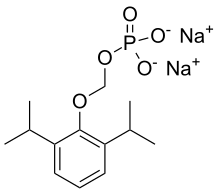
磷丙泊酚
 | |
| 临床资料 | |
|---|---|
| 商品名 | Lusedra |
| AHFS/Drugs.com | Monograph |
| 核准状况 |
|
| 怀孕分级 |
|
| 依赖性 | 未知 |
| 给药途径 | 静脉注射 |
| ATC码 | |
| 法律规范状态 | |
| 法律规范 |
|
| 药物动力学数据 | |
| 血浆蛋白结合率 | 98%[1] |
| 药物代谢 | 肝 葡萄糖醛酸化 |
| 生物半衰期 | 0.81小时[1] |
| 排泄途径 | 肾 |
| 识别信息 | |
| CAS号 | 258516-89-1 258516-87-9(二钠盐) |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| 化学信息 | |
| 化学式 | C13H21O5P |
| 摩尔质量 | 288.28 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
磷丙泊酚(INN[3]:Fospropofol),通常以二钠盐(商品名Lusedra[4])的形式使用,是一种静脉镇静催眠药。它目前被批准用于接受诊断或治疗程序(如内窥镜检查)的成年患者的镇静。
临床应用
广泛使用的静脉麻醉剂丙泊酚(异丙酚)已开发出多种水溶性衍生物和前药,其中磷丙泊酚已被发现最适合临床开发。[5][6]据称,这种水溶性化合物的优点包括静脉注射部位的疼痛减轻、长期给药引起高脂血症的可能性降低以及感染菌血症的几率降低。[来源请求]通常,磷丙泊酚与芬太尼等阿片类药物联合给药。[来源请求]
临床药理学
作用机制
磷丙泊酚是丙泊酚的前体药物;它被碱性磷酸酶代谢为活性代谢物丙泊酚。
药代动力学
研究人员撤回了磷丙泊酚药代动力学的初步试验结果。截至2011年,尚无新结果。[7]
管制物质
在美国的《受控物质法》中,磷丙泊酚被归类为附表IV受控物质。[8]
参考资料
- ^ 1.0 1.1 Eisai Inc. LUSEDRA (fospropofol disodium) Injection (PDF). Woodcliff Lake, New Jersey: Eisai Inc. October 2009 [2 August 2010]. (原始内容 (PDF)存档于22 November 2010).
- ^ Fospropofol disodium. PubChem Compound. Bethesda, Maryland: U.S. National Library of Medicine. [9 February 2017]. (原始内容存档于2023-06-05).
- ^ Recommended INNs 2006, pt 56 (PDF). World Health Organization. World Health Organization. [20 April 2016].
- ^ FDA Approves Fospropofol and Follows ASAs Labeling Recommendation. American Society of Anesthesiologists. 2008-12-15 [2011-03-30]. (原始内容存档于2011-05-26).
- ^ Cooke A, Anderson A, Buchanan K, Byford A, Gemmell D, Hamilton N, et al. Water-soluble propofol analogues with intravenous anaesthetic activity. Bioorganic & Medicinal Chemistry Letters. April 2001, 11 (7): 927–30. PMID 11294393. doi:10.1016/S0960-894X(01)00088-9.
- ^ Bennett DJ, Anderson A, Buchanan K, Byford A, Cooke A, Gemmell DK, et al. Novel water soluble 2,6-dimethoxyphenyl ester derivatives with intravenous anaesthetic activity. Bioorganic & Medicinal Chemistry Letters. June 2003, 13 (12): 1971–5. PMID 12781176. doi:10.1016/S0960-894X(03)00346-9.
- ^ Mahajan B, Kaushal S, Mahajan R. Fospropofol: pharmacokinetics?. Journal of Anaesthesiology Clinical Pharmacology. January 2012, 28 (1): 134–5. PMC 3275955
 . PMID 22345970. doi:10.4103/0970-9185.92472.
. PMID 22345970. doi:10.4103/0970-9185.92472.
- ^ "Schedule of Controlled Substances; Placement of Fospropofol into Schedule IV[永久失效链接]," 74 Federal Register 192 (October 6, 2009), pp. 51234–51236.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
