磷化铝
| 磷化铝 | |
|---|---|
| |
| 别名 | 磷化铝(III) |
| 识别 | |
| CAS号 | 20859-73-8 |
| PubChem | 30332 |
| ChemSpider | 28171 |
| SMILES |
|
| InChI |
|
| InChIKey | PPNXXZIBFHTHDM-LQQCNYPFAR |
| EINECS | 244-088-0 |
| RTECS | BD1400000 |
| 性质 | |
| 化学式 | AlP |
| 摩尔质量 | 58.0 g·mol⁻¹ |
| 外观 | 黄色或灰色晶体 |
| 密度 | 2.42 g/cm³ |
| 熔点 | >1000 °C |
| 溶解性(水) | 可溶 |
| 危险性 | |
| MSDS | External MSDS |
| 欧盟分类 | 有毒 (T) 对环境有害 (N) |
| NFPA 704 | |
| 闪点 | >800 °C |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
磷化铝(英文:aluminum phosphide)是一种化学式为AlP的高毒性无机化合物,可以作为宽能隙的半导体和熏蒸剂。此无色固体在市面上通常会是灰绿色或是灰黄色粉末则是因为水解和氧化产生了杂质。
性质
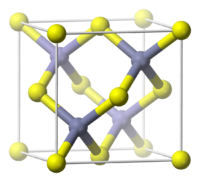
磷化铝晶体的颜色通常为暗灰色或暗黄色,具有闪锌矿晶体结构[1],在300K的晶格常数为5.4510Å[2],在1000 °C(1830°F)以下非常稳定。
- AlP + 3 H2O → Al(OH)3 + PH3
- AlP + 3 H+ → Al3+ + PH3
制备
- 4Al + P4 → 4AlP
要避免磷化铝暴露在任何潮湿的环境下,因为会产生有毒的磷化氢气体。
用途
农药
磷化铝常常用作杀虫剂、杀鼠剂、或是熏蒸剂,以用来储存谷物,它可以用来杀死虫和小型的哺乳动物像是鼹或啮齿目。磷化铝做成的药丸常被称作“麦丸”,通常还包含一些可以增加氨的化学药品,这有助于减少自燃或磷化氢气体爆炸的可能性。
作为杀鼠剂,磷化铝会被混进啮齿目可能会吃的食物中,在啮齿目的消化系统中,与酸反应产生有毒的磷化氢气体。
作为熏蒸剂,它可以放进密闭空间,例如粮仓、船、飞机、甚至是啮齿目的巢穴中,产生有毒的磷化氢气体,成功达到效果。
半导体应用
毒理学
由于磷化铝有毒,它曾被用于自杀。[7]另外熏蒸也可能导致意外死亡,沙特阿拉伯[8]和美国[9] 都曾有过前例,因此有个伊朗法医组织呼吁禁止磷化铝作为农药用途。[10][11] 在西班牙的瓜代拉堡,有一个家庭为了做资源回收而把含有磷化铝的瓶盖收集放在浴室里,结果磷化铝和水反应产生有毒的磷化氢气体,导致家庭成员全部死亡。[12] 另外磷化铝中毒也是印度次大陆的一个大问题。[13][14]
参考资料
- ^ Van Zeghbroeck, B. J. Bravais Lattices; Zincblende Lattice. University of Colorado. 1997 [2015-01-19]. (原始内容存档于2015-01-11).
- ^ Lattice Constants. SiliconFarEast.com. 2004 [10/02/2011]. (原始内容存档于2015-09-24).
- ^ Holleman, Arnold Frederik; Wiberg, Egon, Wiberg, Nils , 编, Inorganic Chemistry, 由Eagleson, Mary; Brewer, William翻译, San Diego/Berlin: Academic Press/De Gruyter, 2001, ISBN 0-12-352651-5
- ^ White, W. E.; Bushey, A. H.; Holtzclaw, H. F.; Hengeveld, F. W. Bailar, J. C. , 编. Aluminum Phosphide. Inorganic Syntheses. 1953, 4: 23–25. doi:10.1002/9780470132357.ch7.
- ^ White, W. E.; Bushey, A. H. Aluminum Phosphide – Preparation and Composition. Journal of the American Chemical Society. 1944, 66 (10): 1666. doi:10.1021/ja01238a018.
- ^ Corbridge, D. E. C. Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology 5th. Amsterdam: Elsevier. 1995. ISBN 0-444-89307-5.
- ^ Millionaire's death sparks poison scare. BBC News. 2002-10-10 [2009-04-05]. (原始内容存档于2012-04-06).
- ^ Fumes kill two Danes in Jeddah. BBC News. 2009-02-24 [2009-02-25]. (原始内容存档于2009年2月25日).
- ^ Family loses 2nd child in suspected pesticide poisoning. KSL-TV. 2010-02-09 [2010-02-09]. (原始内容存档于2010年2月11日).
- ^ S. Shadnia, G. Sasanian, P. Allami, A. Hosseini, A. Ranjbar, N. Amini-Shirazi, M. Abdollahi. A retrospective 7-years study of aluminum phosphide poisoning in Tehran: opportunities for prevention. Human & Experimental Toxicology. 2009-4, 28 (4): 209–213 [2019-02-13]. ISSN 1477-0903. PMID 19734272. doi:10.1177/0960327108097194. (原始内容存档于2019-08-31).
- ^ Mehrpour, O.; Singh, S. Rice Tablet Poisoning: A Major Concern in Iranian Population. Human & Experimental Toxicology. 2010, 29 (8): 701–702. PMID 20097728. doi:10.1177/0960327109359643.
- ^ La familia de Alcalá de Guadaira murió tras inhalar plaguicida. La Vanguardia. Agencia EFE. 3 February 2014 [3 February 2014]. (原始内容存档于2014-02-21).
- ^ Siwach, SB; Gupta, A. The profile of acute poisonings in Harayana-Rohtak Study. The Journal of the Association of Physicians of India. 1995, 43 (11): 756–9. PMID 8773034.
- ^ Singh, D; Jit, I; Tyagi, S. Changing trends in acute poisoning in Chandigarh zone: A 25-year autopsy experience from a tertiary care hospital in northern India. The American journal of forensic medicine and pathology. 1999, 20 (2): 203–10. PMID 10414665. doi:10.1097/00000433-199906000-00019.
| ||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
|
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

