巴氨西林
 | |
| 臨床資料 | |
|---|---|
| 商品名 | Spectrobid(辉瑞) Penglobe(阿斯利康) |
| 其他名稱 | 巴卡西林 巴坎西林 |
| AHFS/Drugs.com | Micromedex详细消费者药物信息 |
| 给药途径 | 口服 |
| ATC碼 | |
| 藥物動力學數據 | |
| 药物代谢 | 迅速水解为氨苄青霉素 |
| 识别信息 | |
| |
| CAS号 | 50972-17-3 37661-08-8 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| 化学信息 | |
| 化学式 | C21H27N3O7S |
| 摩尔质量 | 465.52 g·mol−1 |
| |
巴氨西林(INN:Bacampicillin)是一种青霉素类抗生素。它是氨苄青霉素的前体药物,具有改善的口服生物利用度。[1]
它以Spectrobid(辉瑞)和Penglobe(阿斯利康)的品牌出售。2015年,辉瑞停产了Spectrobid,并且没有仿制药制造商接管生产。[2]因此,巴氨西林在美国不再售卖,并且不再获得FDA批准。[3]
合成
[编辑]氨苄青霉素和苄青霉素之间相对较小的化学差异不仅允许大量的口服活性,而且导致抗微生物谱的显着拓宽,从而允许用于对抗许多革兰氏阴性菌。为了进一步增强氨苄青霉素的口服吸收,已经采用了许多装置。巴氨西林是为此目的而设计的氨苄西林的前体药物。
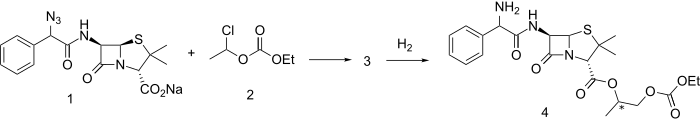
叠氮西林钠盐(1)与混合碳酸酯(2)(本身由乙醛和氯甲酸乙酯制备)反应生成酯(3)。用氢和合适的催化剂还原叠氮基键生成巴氨西林 (4)。两种对映体都有活性。该药物从胃肠道迅速吸收,并迅速被血清酯酶分解为具有生物活性的氨苄青霉素、乙醛、二氧化碳和乙醇。
参考资料
[编辑]- ^ Bodin NO, Ekström B, Forsgren U, Jalar LP, Magni L, Ramsay CH, Sjöberg B. Bacampicillin: a new orally well-absorbed derivative of ampicillin. Antimicrobial Agents and Chemotherapy. November 1975, 8 (5): 518–25. PMC 429411
 . PMID 1211909. doi:10.1128/aac.8.5.518.
. PMID 1211909. doi:10.1128/aac.8.5.518.
- ^ Drugs@FDA: FDA-Approved Drugs , BACAMPICILLIN HYDROCHLORIDE. www.accessdata.fda.gov. [2022-07-29]. (原始内容存档于2023-02-28).
- ^ Organon USA Inc. et al.; Withdrawal of Approval of 67 New Drug Applications and 128 Abbreviated New Drug Applications. unblock.federalregister.gov. [2022-07-29]. (原始内容存档于2021-04-04).
- ^ DE 2311328,Ekström, Bertil; Ödön Kalman Jozsef Kovacs & Berndt Olof Harald Sjöberg,「Penicilline und Verfahren zu deren Herstellung [Penicillin and method for manufacturing thereof]」,发表于1973-10-18
- ^ Ekstrom BA, Kovacs OK, and Sjoberg BO, (1973). Chem. Abstr., 80, 14921q(1974).
- ^ DE 2144457,Ekström, Bertil Ake & Berndt Olof Harald Sjöberg,「α-Aminopenicilline und Verfahren zu deren Herstellung [α-aminopenicillins and processes for their preparation]」,发表于1972-03-30
- ^ Ekstrom BA, Sjoberg BO, 美國專利第3,873,521号 and 美國專利第3,939,270号 (1975 and 1976 both to Astra).
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
