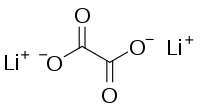
草酸锂
| 草酸锂 | |
|---|---|
| |
| 别名 | 乙二酸锂 |
| 识别 | |
| CAS号 | 553-91-3 |
| PubChem | 68383 |
| ChemSpider | 61669 |
| SMILES |
|
| EINECS | 209-054-1 |
| 性质 | |
| 化学式 | C 2Li 2O 4 |
| 摩尔质量 | 102.0 g·mol⁻¹ |
| 外观 | 无色晶体 |
| 密度 | 2.12 g/cm3 |
| 危险性 | |
GHS危险性符号
| |
| GHS提示词 | 警告 |
| H-术语 | H302, H312 |
| P-术语 | P264, P270, P280, P301+312, P302+352, P312, P322, P330, P363, P501 |
| 相关物质 | |
| 相关化学品 | Calcium oxalate Sodium oxalate Magnesium oxalate Strontium oxalate Barium oxalate Potassium oxalate Beryllium oxalate |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
草酸锂是一种无机化合物,化学式为Li
2C
2O
4,是锂的草酸盐。[1][2]
合成与反应
[编辑]它和草酸铀酰按等化学计量比在溶液中煮沸后冷却,可以得到黄色的Li2UO2(C2O4)2·5H2O晶体。[3]它和五氟化磷反应,得到四氟(草酸根)合磷酸锂(LiPF4C2O4);[4]它和三氟化硼乙醚反应,可以得到相应的硼酸根配合物(LiBF2C2O4)。[5]
参考文献
[编辑]- ^ Beagley, B.; Small, R. W. H. The structure of lithium oxalate. Acta Crystallographica. 1964-06-10, 17 (6): 783–788 [15 June 2021]. doi:10.1107/S0365110X64002079. (原始内容存档于2022-02-04) (英语).
- ^ Solchenbach, Sophie; Wetjen, Morten; Pritzl, Daniel; Schwenke, K. Uta; Gasteiger, Hubert A. Lithium Oxalate as Capacity and Cycle-Life Enhancer in LNMO/Graphite and LNMO/SiG Full Cells. Journal of the Electrochemical Society. 2018, 165 (3): A512–A524 [15 June 2021]. doi:10.1149/2.0611803jes. (原始内容存档于2022-02-04) (英语).
- ^ N.D. Dahale, K.L. Chawla, N.C. Jayadevan, V. Venugopal. X-ray, thermal and infrared spectroscopic studies on lithium and sodium oxalate hydrates. Thermochimica Acta. 1997-06, 293 (1-2): 163–166 [2021-06-16]. doi:10.1016/S0040-6031(97)00015-4. (原始内容存档于2018-06-27) (英语).
- ^ Mengqing Xu, Ang Xiao, Li Yang, Brett Lucht. Novel Electrolyte for Lithium Ion Batteries: Lithum Tetrafluorooxalatophosphate (LiPF4C2O4). ECS Transactions. 2019-12-18, 16 (35): 3–11 [2021-06-16]. ISSN 1938-6737. doi:10.1149/1.3123122.
- ^ Joshua L. Allen, Sang-Don Han, Paul D. Boyle, Wesley A. Henderson. Crystal structure and physical properties of lithium difluoro(oxalato)borate (LiDFOB or LiBF2Ox). Journal of Power Sources. 2011-11, 196 (22): 9737–9742 [2021-06-16]. doi:10.1016/j.jpowsour.2011.07.065. (原始内容存档于2020-02-19) (英语).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

