草酸铍
| 草酸铍 | |
|---|---|
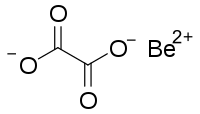
| |
| 识别 | |
| CAS号 | 3173-18-0(无水) 15771-43-4(三水) 15697-24-2(一水) |
| ChemSpider | 4953986 |
| SMILES |
|
| InChI |
|
| InChIKey | XQZGLPVUHKSNBQ-UHFFFAOYSA-L |
| 性质 | |
| 化学式 | C 2BeO 4 |
| 摩尔质量 | 97.03[1] g·mol⁻¹ |
| 外观 | 透明晶体 |
| 沸点 | 365.1 °C(638 K) |
| 溶解性(水) | 63.2 g(三水,25 °C)[2] |
| 相关物质 | |
| 相关化学品 | Calcium oxalate Sodium oxalate Magnesium oxalate Strontium oxalate Barium oxalate Iron(II) oxalate Iron(III) oxalate Lithium oxalate |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
草酸铍是一种无机化合物,化学式为BeC
2O
4,是铍的草酸盐。[3]它是无色晶体,可溶于水,可以形成水合物晶体。它经热分解[4]可以用来制备高纯度的氧化铍。[5]
制备
[编辑]性质
[编辑]草酸铍的水合物加热失水:
需要指出的是,三水合物在50 °C便可缓慢失水,生成一水合物。[7]
参考文献
[编辑]- ^ BERYLLIUM OXALATE. chemicalbook.com. [15 June 2021]. (原始内容存档于2022-02-04).
- ^ Wirth, Fritz. Oxalates and acetates of beryllium. Zeitschrift fuer Anorganische Chemie, 1914. 87: 7-8.
- ^ Novoselova, Aleksandra Vasilʹevna; Bat︠s︡anova, Li︠u︡dmila Rafailovna. Analytical Chemistry of Beryllium. Ann Arbor-Humphrey Science Publishers. 1969: 25 [15 June 2021] (英语).
- ^ Walsh, Kenneth A. Beryllium Chemistry and Processing. ASM International. 2009-01-01: 125 [15 June 2021]. ISBN 978-0-87170-721-5 (英语).
- ^ Dollimore, David; Konieczay, Julie L. The thermal decomposition of beryllium oxalate and related materials. Thermochimica Acta. 1998-09-07, 318 (1–2): 155–163 [15 June 2021]. doi:10.1016/S0040-6031(98)00340-2. (原始内容存档于2022-04-01) (英语).
- ^ Moore, Raymond E. Purification of Beryllium Compounds: A Literature Survey. Oak Ridge National Laboratory. 1960: 6 [15 June 2021] (英语).
- ^ Hamner, R. L.; Harris, L. A. Calcination in air of beryllium oxalate trihydrate to beryllium oxide. United States Atomic Energy Commission [Unclassified and Declassified Reports Published by the Atomic Energy Commission and Its Contractors], 1961. ORNL-3183.
延伸阅读
[编辑]- Sidgwick, Nevil V.; Lewis, Neil B. CLXVI.—The solubility of beryllium oxide in solutions of its salts. J. Chem. Soc. 1926, 129 (0): 1287–1302. ISSN 0368-1769. doi:10.1039/JR9262901287.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

![{\displaystyle {\mathsf {BeC_{2}O_{4}\cdot 3H_{2}O\ {\xrightarrow[{-2H_{2}O}]{100^{o}C))\ BeC_{2}O_{4}\cdot H_{2}O\ {\xrightarrow[{-H_{2}O}]{220^{o}C))\ BeC_{2}O_{4))))](https://wikimedia.org/api/rest_v1/media/math/render/svg/26f1ccf7450ff492ba682cba56b28df05ac0b8c3)
