磷化铝
| 磷化铝 | |
|---|---|
| |
| 别名 | 磷化铝(III) |
| 识别 | |
| CAS号 | 20859-73-8 |
| PubChem | 30332 |
| ChemSpider | 28171 |
| SMILES |
|
| InChI |
|
| InChIKey | PPNXXZIBFHTHDM-LQQCNYPFAR |
| EINECS | 244-088-0 |
| RTECS | BD1400000 |
| 性质 | |
| 化学式 | AlP |
| 摩尔质量 | 58.0 g·mol⁻¹ |
| 外观 | 黄色或灰色晶体 |
| 密度 | 2.42 g/cm³ |
| 熔点 | >1000 °C |
| 溶解性(水) | 可溶 |
| 危险性 | |
| MSDS | External MSDS |
| 欧盟分类 | 有毒 (T) 对环境有害 (N) |
| NFPA 704 | |
| 闪点 | >800 °C |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
磷化鋁(英文:aluminum phosphide)是一種化學式為AlP的高毒性無機化合物,可以作為寬能隙的半導體和熏蒸劑。此無色固體在市面上通常會是灰綠色或是灰黃色粉末則是因為水解和氧化產生了雜質。
性質
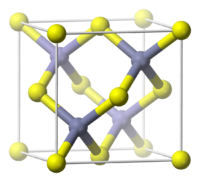
[编辑]磷化鋁晶體的顏色通常為暗灰色或暗黃色,具有閃鋅礦晶體結構[1],在300K的晶格常數為5.4510Å[2],在1000 °C(1830°F)以下非常穩定。
- AlP + 3 H2O → Al(OH)3 + PH3
- AlP + 3 H+ → Al3+ + PH3
製備
[编辑]- 4Al + P4 → 4AlP
要避免磷化鋁暴露在任何潮濕的環境下,因為會產生有毒的磷化氫氣體。
用途
[编辑]農藥
[编辑]磷化鋁常常用作殺蟲劑、殺鼠劑、或是熏蒸劑,以用來儲存穀物,它可以用來殺死蟲和小型的哺乳動物像是鼴或齧齒目。磷化鋁做成的藥丸常被稱作“麥丸”,通常還包含一些可以增加氨的化學藥品,這有助於減少自燃或磷化氫氣體爆炸的可能性。
作為殺鼠劑,磷化鋁會被混進齧齒目可能會吃的食物中,在齧齒目的消化系統中,與酸反應產生有毒的磷化氫氣體。
作為熏蒸劑,它可以放進密閉空間,例如糧倉、船、飛機、甚至是齧齒目的巢穴中,產生有毒的磷化氫氣體,成功達到效果。
半導體應用
[编辑]在工業中,磷化鋁是一種半導體材料,通常會和其他二元材料形成合金,以作為發光二極體(LED),例如:鋁鎵磷化銦。[6]
毒理學
[编辑]由于磷化鋁有毒,它曾被用於自殺。[7]另外熏蒸也可能導致意外死亡,沙烏地阿拉伯[8]和美國[9] 都曾有過前例,因此有個伊朗法醫組織呼籲禁止磷化鋁作為農藥用途。[10][11] 在西班牙的瓜代拉堡,有一個家庭為了做資源回收而把含有磷化鋁的瓶蓋收集放在浴室裡,結果磷化鋁和水反應產生有毒的磷化氫氣體,導致家庭成員全部死亡。[12] 另外磷化鋁中毒也是印度次大陸的一個大問題。[13][14]
參考資料
[编辑]- ^ Van Zeghbroeck, B. J. Bravais Lattices; Zincblende Lattice. University of Colorado. 1997 [2015-01-19]. (原始内容存档于2015-01-11).
- ^ Lattice Constants. SiliconFarEast.com. 2004 [10/02/2011]. (原始内容存档于2015-09-24).
- ^ Holleman, Arnold Frederik; Wiberg, Egon, Wiberg, Nils , 编, Inorganic Chemistry, 由Eagleson, Mary; Brewer, William翻译, San Diego/Berlin: Academic Press/De Gruyter, 2001, ISBN 0-12-352651-5
- ^ White, W. E.; Bushey, A. H.; Holtzclaw, H. F.; Hengeveld, F. W. Bailar, J. C. , 编. Aluminum Phosphide. Inorganic Syntheses. 1953, 4: 23–25. doi:10.1002/9780470132357.ch7.
- ^ White, W. E.; Bushey, A. H. Aluminum Phosphide – Preparation and Composition. Journal of the American Chemical Society. 1944, 66 (10): 1666. doi:10.1021/ja01238a018.
- ^ Corbridge, D. E. C. Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology 5th. Amsterdam: Elsevier. 1995. ISBN 0-444-89307-5.
- ^ Millionaire's death sparks poison scare. BBC News. 2002-10-10 [2009-04-05]. (原始内容存档于2012-04-06).
- ^ Fumes kill two Danes in Jeddah. BBC News. 2009-02-24 [2009-02-25]. (原始内容存档于2009年2月25日).
- ^ Family loses 2nd child in suspected pesticide poisoning. KSL-TV. 2010-02-09 [2010-02-09]. (原始内容存档于2010年2月11日).
- ^ S. Shadnia, G. Sasanian, P. Allami, A. Hosseini, A. Ranjbar, N. Amini-Shirazi, M. Abdollahi. A retrospective 7-years study of aluminum phosphide poisoning in Tehran: opportunities for prevention. Human & Experimental Toxicology. 2009-4, 28 (4): 209–213 [2019-02-13]. ISSN 1477-0903. PMID 19734272. doi:10.1177/0960327108097194. (原始内容存档于2019-08-31).
- ^ Mehrpour, O.; Singh, S. Rice Tablet Poisoning: A Major Concern in Iranian Population. Human & Experimental Toxicology. 2010, 29 (8): 701–702. PMID 20097728. doi:10.1177/0960327109359643.
- ^ La familia de Alcalá de Guadaira murió tras inhalar plaguicida. La Vanguardia. Agencia EFE. 3 February 2014 [3 February 2014]. (原始内容存档于2014-02-21).
- ^ Siwach, SB; Gupta, A. The profile of acute poisonings in Harayana-Rohtak Study. The Journal of the Association of Physicians of India. 1995, 43 (11): 756–9. PMID 8773034.
- ^ Singh, D; Jit, I; Tyagi, S. Changing trends in acute poisoning in Chandigarh zone: A 25-year autopsy experience from a tertiary care hospital in northern India. The American journal of forensic medicine and pathology. 1999, 20 (2): 203–10. PMID 10414665. doi:10.1097/00000433-199906000-00019.
| ||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
|
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

