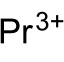磷化镨
| 磷化镨 | ||
|---|---|---|
|
| ||
| 别名 | Phosphanylidynepraseodymium | |
| 识别 | ||
| CAS号 | 12066-49-8 | |
| PubChem | 82904 | |
| ChemSpider | 74805 | |
| SMILES |
| |
| EINECS | 235-068-2 | |
| 性质 | ||
| 化学式 | PPr | |
| 摩尔质量 | 171.88 g·mol−1 | |
| 外观 | 深绿色晶体[1] | |
| 密度 | g/cm3 | |
| 溶解性(水) | 在水中分解 | |
| 结构 | ||
| 晶体结构 | 立方 | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | ||
磷化镨是镨和磷的无机化合物,化学式为PrP。[2][3][4]该化合物会结晶。
合成
[编辑]在碘蒸汽下将镨金属和磷共热:[5]
物理性质
[编辑]磷化镨会形成立方晶体,空间群为F m3m,晶格常数为a = 0.5872 nm,Z = 4,结构类似氯化钠NaCl。[6][7]
这个化合物在3120 °C均匀融化。
参考文献
[编辑]- ^ Rowley, Adrian T.; Parkin, Ivan P. Convenient synthesis of lanthanide and mixed lanthanide phosphides by solid-state routes involving sodium phosphide. Journal of Materials Chemistry (Royal Society of Chemistry (RSC)). 1993, 3 (7): 689. ISSN 0959-9428. doi:10.1039/jm9930300689.
- ^ Praseodymium Phosphide. American Elements. [14 December 2021]. (原始内容存档于2021-12-14) (英语).
- ^ Toxic Substances Control Act (TSCA) Chemical Substance Inventory. Cumulative Supplement to the Initial Inventory: User Guide and Indices. United States Environmental Protection Agency. 1980: 252 [14 December 2021] (英语).
- ^ O'Bannon, Loran. Dictionary of Ceramic Science and Engineering. Springer Science & Business Media. 6 December 2012: 199 [14 December 2021]. ISBN 978-1-4613-2655-7 (英语).
- ^ Mironov, K. E. A transport reaction for the growth of praseodymium phospide. Journal of Crystal Growth. 1 January 1968, 3–4: 150–152 [14 December 2021]. Bibcode:1968JCrGr...3..150M. doi:10.1016/0022-0248(68)90115-2 (英语).
- ^ Nowacki, J. D. H. Donnay, and Werner. Crystal Data: Classification of Substances by Space Groups and their Identification from Cell Dimensions. Geological Society of America. 1954: 509 [14 December 2021]. ISBN 978-0-8137-1060-0 (英语).
- ^ Yaduvanshi, Namrata; Singh, Sadhna. Exploration of pressure induced phase transition in praseodymium phosphide (PrP) with the NaCl-type structure. Dae Solid State Physics Symposium 2017. AIP Conference Proceedings 1942 (1): 030001. 10 April 2018 [14 December 2021]. Bibcode:2018AIPC.1942c0001Y. doi:10.1063/1.5028582. (原始内容存档于2021-12-14).
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.