二异丁基氢化铝
| 二异丁基氢化铝 | |
|---|---|
| |

| |
| IUPAC名 Diisobutylaluminium hydride | |
| 别名 | DIBAH、DIBAL、DiBAlH、DIBAL-H、DIBALH |
| 识别 | |
| CAS号 | 1191-15-7 |
| ChemSpider | 10430352 |
| SMILES |
|
| InChI |
|
| InChIKey | AZWXAPCAJCYGIA-DFAADSFOAF |
| 性质 | |
| 化学式 | C16H38Al2 (dimer) |
| 摩尔质量 | (单体)142.22 g·mol⁻¹ |
| 外观 | 无色液体 |
| 密度 | 0.798 g/cm3 |
| 熔点 | –18 °C |
| 沸点 | 116–118 °C/1 mmHg |
| 危险性 | |
| 主要危害 | 在空气中自燃 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
二异丁基氢化铝(DIBAL、DIBAL-H、DIBAH)是有机合成中常用的有机金属还原剂之一,化学式(i-Bu2AlH)2,室温下为无色液体。由烯烃聚合反应的共催化性质而被研究,[1]一般以它溶于有机溶剂(如甲苯)中的形式出售。
它由三丁基铝120-180°C下进行减压热分解制得(β-氢消除反应),副产物为三异丁基铝,可回收使用:[2]
- (i-Bu3Al)2 → (i-Bu2AlH)2 + 2 (CH3)2C=CH2
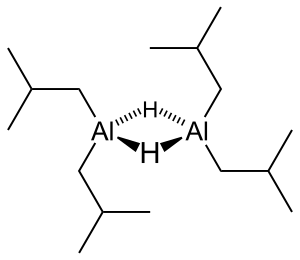
温度不可过高,否则产物会分解为金属铝。X射线晶体学的数据表明,二异丁基氢化铝存在二聚体(见图)和三聚体,铝为四面体结构,以桥连的氢原子相连。[3]
它在有机合成用作亲电性的温和还原剂,可将酯、腈还原为醛,酰胺还原为醛或胺,羧酸、酰卤还原为醇,α,β-不饱和酯还原为烯丙醇。[4]它与水迅速反应生成氢和异丁烷。
参见
[编辑]参考资料
[编辑]- ^ K. Ziegler, H. Martin and F. Krupp. Metallorganische Verbindungen, XXVII Aluminiumtrialkyle und Dialkyl-Aluminiumhydride Aus Aluminiumisobutyl-Verbindungen. Justus Liebigs Annalen der Chemie. 1960, 629 (1): 14–19. doi:10.1002/jlac.19606290103.
- ^ Eisch, J. J. Organometallic Syntheses Volume 2, Academic Press: New York, 1981. ISBN 0-12-234950-4.
- ^ Mark F. Self, William T. Pennington and Gregory H. Robinson "Reaction of diisobutylaluminum hydride with a macrocyclic tetradentate secondary amine. Synthesis and molecular structure of [Al(iso-Bu)]2[C10H20N4][Al(iso-Bu)3]2: evidence of an unusual disproportionation of (iso-Bu)2AlH" Inorganica Chimica Acta (1990), volume 175, pp. 151-3. doi:10.1016/S0020-1693(00)84819-7.
- ^ Galatsis, P. “Diisobutylaluminum Hydride” in Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons: New York, 2001. doi:10.1002/047084289X.rd245
外部链接
[编辑]Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
