亚氯酸银
| 亚氯酸银 | |
|---|---|
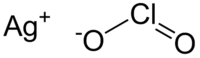
| |
| IUPAC名 Silver chlorite | |
| 英文名 | Silver chlorite |
| 别名 | 亚氯酸银(I) |
| 识别 | |
| CAS号 | 7783-91-7 |
| PubChem | 9855611 |
| ChemSpider | 8031311 |
| SMILES |
|
| InChI |
|
| 性质 | |
| 化学式 | AgClO2 |
| 摩尔质量 | 175.32 g·mol−1 |
| 外观 | 浅黄色固体 |
| 熔点 | 156 °C(429 K)(分解[2]) |
| 溶解性(水) | 0.45 g/100ml[1] |
| 折光度n D |
2.1[2] |
| 结构[3] | |
| 晶体结构 | 正交晶系 |
| 空间群 | Pcca |
| 晶格常数 | a = 6.075 Å, b = 6.689 Å, c = 6.123 Å |
| 热力学 | |
| ΔfHm⦵298K | 0.0 kcal/mol[1] |
| S⦵298K | 32.16 cal/deg[4] |
| 热容 | 20.81 cal/deg[4] |
| 危险性 | |
GHS危险性符号
| |
| 相关物质 | |
| 其他阴离子 | 氯酸银 高氯酸银 |
| 其他阳离子 | 亚氯酸钠 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
亚氯酸银是一种无机化合物,化学式 AgClO2。这种正交晶系的微黄色固体对冲击敏感。
制备
- AgNO
3 + NaClO
2 → AgClO
2 + NaNO
3
反应和性质
如果正常加热到 105 °C,亚氯酸银就会剧烈爆炸:[2]
- AgClO
2 → AgCl + O
2
如果非常小心地加热,它到 156 °C 才会分解成氯化银。如果有亚氯酸存在,会产生氯酸银。[2]
亚氯酸银会和如硫和盐酸等各种物质爆炸性反应,形成氯化银。它也会被二氧化硫还原,和硫酸反应产生二氧化氯。[6]亚氯酸银和碘甲烷或碘乙烷反应会爆炸。[7]
配合物
- AgClO
2 + 3NH
3 → 3NH
3·AgClO
2
参考资料
- ^ 1.0 1.1 A. G. Massey; N. R. Thompson; B. F. G. Johnson. The Chemistry of Copper, Silver and Gold (Ebook). Pergamon International Library of Science, Technology, Engineering and Social Studies: Elsevier Science. 2016: 108. ISBN 9781483181691 (英语).
- ^ 2.0 2.1 2.2 2.3 F. Solymosi. The Thermal Stability and Some Physical Properties of Silver Chlorite, Chlorate and Perchlorate*. Zeitschrift für Physikalische Chemie (Oldenbourg Wissenschaftsverlag). 1968, 57 (1): 1–18. doi:10.1524/zpch.1968.57.1_2.001 (英语).
- ^ M. Okuda; M. Ishihara; M. Yamanaka; S. Ohba; Y. Saito. Structures of lead chlorite, magnesium chlorite hexahydrate and silver chlorite. Acta Cryst. 1990, 46: 1755–1759. doi:10.1107/S010827019000066X.
- ^ 4.0 4.1 Wendell V. Smith; Kenneth S. Pitzer; Wendell M. Latimer. Silver Chlorite: Its Heat Capacity from 15 to 300°K., Free Energy and Heat of Solution and Entropy. The Entropy of Chlorite Ion. J. Am. Chem. Soc. 1937, 59 (12): 2640–2642. doi:10.1021/ja01291a046 (英语).
- ^ J. Cooper; R. E. Marsh. On the structure of AgClO2. Acta Cryst.: 202–203. doi:10.1107/S0365110X61000693 (英语).
- ^ 6.0 6.1 Joseph William Mellor. Supplement to Mellor's Comprehensive Treatise on Inorganic and Theoretical Chemistry: suppl. 3. K, Rb, Cs, Fr. University of Illinois at Urbana-Champaign: Longmans, Green and Company. 1922: 284 (英语).
- ^ Urben, Peter (编). Bretherick's Handbook of Reactive Chemical Hazards. Elsevier Science. 2013: 4. ISBN 9780080523408 (英语).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
