氟化锌
| 氟化锌 | |
|---|---|
| |

| |
| 英文名 | Zinc fluoride |
| 别名 | 二氟化锌 |
| 识别 | |
| CAS号 | 7783-49-5 13986-18-0(四水) |
| PubChem | 24551 |
| ChemSpider | 22957 |
| SMILES |
|
| InChI |
|
| InChIKey | BHHYHSUAOQUXJK-NUQVWONBAR |
| EINECS | 232-001-9 |
| RTECS | ZH3200000 |
| 性质 | |
| 化学式 | ZnF2 |
| 摩尔质量 | 103.406 g/mol(无水) 175.45 g/mol(四水) |
| 外观 | 无色针状晶体或白色粉末 |
| 密度 | 4.95 g/cm3(无水) 2.30 g/cm3(四水) |
| 熔点 | 872 °C(无水) 100 °C 分解(四水) |
| 沸点 | 1500 °C(无水) |
| 溶解性(水) | 0.000052 g/100 mL(无水) 1.52 g/100 mL, 20 °C(四水) |
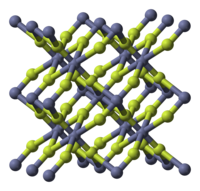
| 结构 | |
| 晶体结构 | 四方晶系(无水),tP6 |
| 空间群 | P42/mnm(No. 136 ) |
| 危险性 | |
| 欧盟危险性符号 | |
| 警示术语 | R:R36/37/38 |
| 安全术语 | S:S26, S37/39 |
| NFPA 704 | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
性质
[编辑]无色针状晶体或白色粉末。有无水物和四水合物两种。与其他卤化锌不同,有明显的离子键化合物特性:熔点很高,溶解度也较小。金红石型结构,锌为六配位。[1] 四水合物为菱方晶系。[2] 结晶体能使光强烈地偏振。
难溶于水,几乎完全不溶于液氨和乙醇,微溶于稀氢氟酸,略溶于盐酸、硝酸和氨水。水溶液很易水解,已知生成的碱式盐为 Zn(OH)F。[3]
与氢气共热时被还原:
制取
[编辑]1、由氢氟酸与碳酸锌(或氢氧化锌)作用,然后蒸发浓缩,得到氟化锌四水合物。将它在减压下加热,就得到无水物。
用途
[编辑]含有部分结晶水的氟化锌是一种很有价值的中等的荧光剂,但是无水氟化锌却无此特性。工业上氟化锌用作陶瓷的釉和涂料、催化剂和木材的防水剂。[4] 也用于有机化合物的氟化反应。
参见
[编辑]参考资料
[编辑]- ^ 1.0 1.1 Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements 2nd. Oxford:Butterworth-Heinemann. 1997. ISBN 0-7506-3365-4.
- ^ Dale L. Perry, Sidney L. Phillips, 1995, Handbook of Inorganic Compounds, CRC Press, ISBN 0849386713
- ^ O. K. Srivastava, E. A. Secco. Studies on metal hydroxy compounds. I. Thermal analyses of zinc derivatives ε-Zn(0H)2, Zn5(OH)8Cl2.H2O, β-ZnOHC1, and ZnOHF. Canadian Journal of Chemistry. 1967: 579–583.
- ^ 吕云阳,王文绍,刘颂禹,季振平. 《无机化学丛书》第六卷:卤素、铜分族、锌分族. 北京: 科学出版社. 1995年12月: 706. ISBN 7-03-003647-6.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.







