氟化铅
| 氟化铅 | |
|---|---|

| |
| |
| 别名 | 二氟化铅 |
| 识别 | |
| CAS号 | 7783-46-2 |
| PubChem | 24549 |
| 性质 | |
| 化学式 | PbF2 |
| 摩尔质量 | 245.20 g·mol⁻¹ |
| 外观 | 白色粉末 |
| 氣味 | 無味 |
| 密度 | 8.445 g/cm3 (orthorhombic) 7.750 g/cm3 (cubic) |
| 熔点 | 824°C |
| 沸点 | 1293°C |
| 溶解性(水) | 0.057 g/100 mL (0 °C) 0.0671 g/100 mL (20 °C)[1] |
| 溶度积Ksp | 2.05 x 10-8 (20 °C) |
| 溶解性 | 可溶於硝酸中 不溶於丙酮及氨 |
| 结构 | |
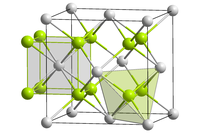
| 晶体结构 | 萤石(cubic),cF12 |
| 空间群 | Fm3m, No. 225 |
| 危险性 | |
| 致死量或浓度: | |
LD50(中位剂量)
|
3031 mg/kg(大鼠口服) |
| 相关物质 | |
| 其他阴离子 | 氯化铅 溴化铅 碘化鉛 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
氟化铅(Lead(II) fluoride)化學式為PbF2,是白色固體。氟化铅有同质异形体,在常溫下為正交晶系(PbCl2型),在高溫下則是立方晶系(Fluorite type)[2]。
製備
[编辑]氟化铅可以用多種方式製備,可以將氫氧化鉛或碳酸鉛和氫氟酸反應,再將生成物中的水蒸發而得[3]:
- Pb(OH)2 + 2 HF → PbF2 + 2 H2O
氟化铅也可以用在铅鹽溶液中加入氫氟酸,或是在硝酸鉛溶液中加入氟化鉀[4]:
- 2 KF + Pb(NO3)2 → PbF2 + 2 KNO3
- 2 NaF + Pb(CH3COO)2 → PbF2 + 2 NaCH3COO
用途
[编辑]氟化铅可以用在低熔點的玻璃、可以反應紅外線的玻璃塗層、CRT電視的磷光體,以及製造甲基吡啶時的催化劑[3]。缪子g-2粒子物理實驗就是用PbF
2閃爍體探測器配合矽的光電倍增器[5]。
參考資料
[编辑]- ^ NIST-data review 1980
- ^ Haines, J.; Léger, J. M.; Schulte, O. High-pressure isosymmetric phase transition in orthorhombic lead fluoride. Physical Review B (American Physical Society (APS)). 1998-04-01, 57 (13): 7551–7555. Bibcode:1998PhRvB..57.7551H. ISSN 0163-1829. doi:10.1103/physrevb.57.7551.
- ^ 3.0 3.1 Carr, Dodd S., Lead Compounds, Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, 2005, doi:10.1002/14356007.a15_249
- ^ Arnold Hollemann, Egon Wiberg, 101st ed., de Gruyter 1995 Berlin; ISBN 3-11-012641-9
- ^ Grange, J.; et al. Muon (g−2) Technical Design Report. Jan 27, 2015. Bibcode:2015arXiv150106858G. arXiv:1501.06858
 . 已忽略未知参数
. 已忽略未知参数|collaboration=(帮助) Via inSPIRE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
