氮族元素的氢化物
氮族元素的氢化物,又称磷属化氢[1](英语:hydrogen pnictides)是由氢原子与氮族元素原子(氮、磷、砷、锑、铋或镆)构成的化合物,是一种氢二元化合物。
氮族元素的三氢化物
最简单的氮族元素氢化物由三个氢原子与一个氮族元素原子化合而成,其化学式通常计为XH3,少数情况下会计做H3X,其中X表示任何一种氮族元素。这些分子的结构都是三角锥形分子构型(有别于硼族元素的三氢化物的平面三角形分子构型)且皆具有极性。
这些三氢化物最稳定的是氨,而位于周期表越下方的越不稳定,且具有毒性。
| 化合物 | 化学式 | 键长 | 空间填充模型 |
|---|---|---|---|
氨 胺 |
 [2] [2] |
 | |
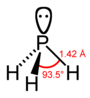
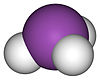
膦 |
 |
 | |
胂 |
 |
 | |
䏲 |
 |
 | |
䏟 |
 |
 | |
氮族元素的三氢化物的性质如下[4]:
| 性质 | 氮化氢(氨) | 磷化氢(膦) | 砷化氢(胂) | 锑化氢(䏲) | 铋化氢(䏟) |
|---|---|---|---|---|---|
| 化学式 | NH3 | PH3 | AsH3 | SbH3 | BiH3 |
| 熔点 (°C) | −77.8 | −133.5 | −116.3 | −88 | ? |
| 沸点 (°C) | −34.5 | −87.5 | −62.4 | −18.4 | 16.8 (推测) |
| 液体密度 (g/cm3) | 0.683 (−34 °C) | 0.746 (−90 °C) | 1.640 (−64 °C) | 2.204 (−18 °C) | ? |
| ΔH° f/kJ mol−1 |
−46.1 | +5[5] | +66.4 | +145.1 | +277.8 |
| 键长 (X–H)/pm | 101.7 | 141.9 | 151.9 | 170.7 | 177.59 |
| 键角 H–X–H | 107.8° | 93.6° | 91.8° | 91.3° | 90.48°[6] |
氮族元素四氢化物离子
氮族元素三氢化物还可以再被质子化成化学式形如XH4+的𬭩离子,且为四面体形分子:
| 离子 | 化学式 | 分子构型 | pka | 分子结构图 | 球-棍模型 | 有机衍生物 |
|---|---|---|---|---|---|---|
铵 |
四面体型 | 9.25[7] |  |
 |
季铵盐 | |
𬭸 |
四面体型 |  |
 |
季𬭸盐 | ||
𬬹 |
四面体型[8] |  |
 |
|||
四氢锑阳离子 |
四面体型[8] |
-2价氮族元素的氢化物
另一种常见的氮族元素氢化物为2个氮族元素原子和四个氢原子化合而成,常见的比如由氮构成的联氨,或称肼。
| 化合物 | 化学式 | 分子结构图 | 空间填充模型 | 质子化物种 |
|---|---|---|---|---|
肼 联氨 (联胺) |
 |
 |
N2H+ 5 N2H2+ 6 | |
联膦 |
 |
 |
||
联胂 |
 |
 |
参见
参考文献
- ^ 磷屬化物 pnictide. 国家教育研究院. [2017-07-21]. (原始内容存档于2017-08-10).
- ^ ammonia. pubchem. [2017-07-21]. (原始内容存档于2017-06-20).
- ^ Fricke, Burkhard. Superheavy elements: a prediction of their chemical and physical properties. Recent Impact of Physics on Inorganic Chemistry. 1975, 21: 89–144 [2013-10-04]. doi:10.1007/BFb0116498. (原始内容存档于2015-04-18).
- ^ 4.0 4.1 Greenwood, Norman Neill; Earnshaw, Alan. Chemistry of the elements. 2016. ISBN 978-0-7506-3365-9. OCLC 1040112384 (英语)., pp. 557–8
- ^ Zumdahl, Steven S. Chemical Principles 6th. Houghton Mifflin. 2009: A22. ISBN 978-0-618-94690-7.
- ^ W. Jerzembeck, H. Bürger, L. Constantin, L. Margulès, J. Demaison, J. Breidung, W. Thiel. Bismuthine BiH3: Fact or Fiction? High-Resolution Infrared, Millimeter-Wave, and Ab Initio Studies. Angew. Chem. Int. Ed. 2002, 41 (14): 2550–2552 [2017-08-10]. (原始内容存档于2013-01-05).
- ^ Garrett, R.H. and Grisham, C.M. Biochemistry. Cengage Learning. 2016: 43 [2017-08-10]. ISBN 9781305886896. (原始内容存档于2017-08-10).
- ^ 8.0 8.1 R. Minkwitz, R.; Kornath, A.; Sawodny, W.; Härtner, H. Über die Darstellung der Pnikogenoniumsalze AsH4+SbF6−, AsH4+AsF6−, SbH4+SbF6−. Zeitschrift für anorganische und allgemeine Chemie. 1994, 620 (4): 753–756. doi:10.1002/zaac.19946200429.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.



