四溴化钛
| 四溴化钛 | |
|---|---|
| |
| |
| IUPAC名 Titanium(IV) bromide | |
| 英文名 | Titanium tetrabromide |
| 别名 | 溴化钛、溴化钛(IV) |
| 识别 | |
| CAS号 | 7789-68-6 |
| PubChem | 123263 |
| SMILES |
|
| InChI |
|
| EINECS | 232-185-0 |
| 性质 | |
| 化学式 | TiBr4 |
| 摩尔质量 | 367.483 g·mol⁻¹ |
| 外观 | 琥珀色固体[1] |
| 密度 | 3.25 g/cm3[2] |
| 熔点 | 39.5 °C(312.6 K)[3] |
| 沸点 | 233.4 °C(506.5 K)[4] |
| 溶解性(水) | 水解[1] |
| 溶解性 | 易溶于醇[1] 可溶于醚[1] 可溶于浓盐酸[1] |
| 结构 | |
| 晶体结构 | 立方晶系, Pa3, Z = 8 |
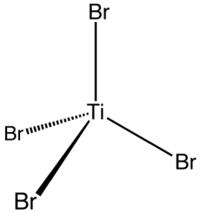
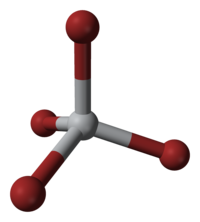
| 配位几何 | 四面体 |
| 偶极矩 | 0 D |
| 危险性 | |
| 警示术语 | R:14-34 |
| 安全术语 | S:26-36/37/39-45 |
| 主要危害 | 腐蚀性 |
| NFPA 704 | |
| 闪点 | 不可燃 |
| 相关物质 | |
| 其他阴离子 | 四氯化钛 四氟化钛 四碘化钛 |
| 相关化学品 | 三溴化钛 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
四溴化钛的化学式是TiBr4,其性质介于四碘化钛和四氯化钛之间。这类4价钛的四配物有些关键的通性,如路易士酸性较高,且易溶于非极性有机溶剂。TiBr4为抗磁性物质,中心原子的外层电子组态为d0。[5]
制备
四溴化钛也可以由以下反应产生:[1]
性质
四溴化钛是一种琥珀黄色晶体。它极易潮解,会水解成二氧化钛和氢溴酸。刚结晶的四溴化钛是四碘化锡结构的,空间群 Pa3 (No. 205),晶格参数 a = 1130.0 pm。放置很久的四溴化钛会转变成四溴化锡结构,空间群 P21/c (No. 14),晶格参数 a = 1017 pm、b = 709 pm、c = 1041 pm 和β = 101.97°。[1][6]
反应
四溴化钛会形成加合物,如 TiBr4(THF)2 和 [TiBr5]−。[7] 它和2-甲基吡啶 (2-Mepy)配合,形成五配位的 TiBr4(2-MePy)。这种分子是三角双锥构型的,吡啶环在水平方向。[8]
四溴化钛和四氯化钛混合,会形成各种通式 TiBr4−xClx (x = 0-4)的化合物。它的机理不明。[10]
参考文献
- ^ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Georg Brauer (Hrsg.), unter Mitarbeit von Marianne Baudler u. a.: Handbuch der Präparativen Anorganischen Chemie. 3., umgearbeitete Auflage. Band II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3, S. 1348ff.
- ^ 来源:Sigma-Aldrich Co., product no. (({id))} .
- ^ Fritsch, Pierre. Magnetic rotatory power of TiBr4. Compt. rend., 1943. 217: 447.
- ^ Rolf B. Johannesen, Charles L. Gordon, Karl H. Gayer, Lawrence Balash. S. Y. Tyree , 编. Titanium(IV) Bromide. Hoboken, NJ, USA: John Wiley & Sons, Inc. 2007-01-05: 46–50 [2021-01-12]. ISBN 9780470132401. doi:10.1002/9780470132401.ch14.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Jean D'Ans, Ellen Lax, [《四溴化钛》在Google Books的内容。 Taschenbuch für Chemiker und Physiker], Springer DE. 1997: pp. 766, (德文)
- ^ Colin S. Creaser & J. Alan Creighton. Pentachloro- and pentabromo-titanate(IV) ions. J. Chem. Soc., Dalton Trans. 1975, (14): 1402–1405. doi:10.1039/DT9750001402.
- ^ Hensen, K.; Lemke, A.; Bolte, M. Tetrabromo(2-methylpyridine-N)-titanate(IV). Acta Crystallographica. 2000, C56 (12): e565–e566. doi:10.1107/S0108270100015407.
- ^ B. Patterson, S. Marumoto & S. D. Rychnovsky. Titanium(IV)-Promoted Mukaiyama Aldol-Prins Cyclizations. Org. Lett. 2003, 5 (17): 3163–3166. PMID 12917007. doi:10.1021/ol035303n.
- ^ S. P. Webb & M. S. Gordon. Intermolecular Self-Interactions of the Titanium Tetrahalides TiX4 (X = F, Cl, Br). J. Am. Chem. Soc. 1999, 121 (11): 2552–2560 [2021-07-17]. doi:10.1021/ja983339i. (原始内容存档于2021-05-15).
| ||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.






