溴化亚铜
| 溴化亚铜 | |
|---|---|

| |
| |
| IUPAC名 Copper(I) bromide 溴化铜(I) | |
| 识别 | |
| CAS号 | 7787-70-4 |
| PubChem | 24593 |
| ChemSpider | 22995 |
| SMILES |
|
| InChI |
|
| InChIKey | NKNDPYCGAZPOFS-REWHXWOFAY |
| 性质 | |
| 化学式 | CuBr |
| 摩尔质量 | 143.45 g·mol⁻¹ |
| 外观 | 无色粉末,不纯时为绿色 |
| 密度 | 4.71 g/cm3(固) |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
溴化亚铜(化学式:CuBr)是铜(I)化合物之一。无色反磁性粉末,易受氧化而呈铜(II)的绿色。[1] 难溶于水,主要用作有机合成试剂。
制备
溴化亚铜常由溴离子存在下二价铜盐被亚硫酸根离子还原得到,方法与氯化亚铜的制备过程类似。[2]
金属铜与溴蒸汽在加热时反应,得到溴化铜与溴化亚铜的混合物。如果金属铜为粉状,则反应在室温就可发生。
铜粉溶于氢溴酸时也可生成溴化亚铜:
- 2 HBr + 2 Cu → 2 CuBr + H2
性质

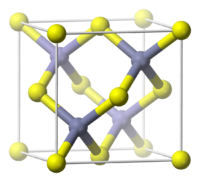
溴化亚铜难溶于水和大多数溶剂,但易溶于盐酸、氢溴酸和氨水。它可被氧化剂氧化为Cu2+,也可被还原剂(如硫酸亚铁)还原为铜。室温时为闪锌矿结构,铜以四面体与四个溴配位,加热时则依次转变为纤锌矿和氯化钠型结构。
溴化亚铜可以与软的路易斯碱形成加合物,例如与二甲基硫醚反应时,会形成无色的CuBr(S(CH3)2)。[3] 该配合物是制取其他有机铜化合物的原料。铜在其中为二配位的直线型结构。
- CuBr + S(CH3)2 → CuBr(S(CH3)2)
溴化亚铜是Sandmeyer反应中的溴化试剂,用于将芳香重氮盐转化为溴化物:[4]
- ArN2+ + CuBr → ArBr + N2 + Cu+
参考资料
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Keller, R. N.; Wycoff, H. D. "Copper(I) chloride" Inorganic Syntheses 1946; Volume II, p 1-4.
- ^ Jarowicki, K.; Kocienski, P. J.; Qun, L. "1,2-Metallate Rearrangement: (Z)-4-(2-Propenyl)-3-Octen-1-ol" Organic Syntheses, Collected Volume 10, p.662 (2004).http://www.orgsyn.org/orgsyn/pdfs/V79P0011.pdf
- ^ Hartwell, J. L. "o-Chlorobromobenzene" Organic Syntheses, Collected Volume 3, p.185 (1955).http://www.orgsyn.org/orgsyn/pdfs/CV3P0185.pdf (页面存档备份,存于互联网档案馆)
| ||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
