磷酸亚铁
| 磷酸亚铁 | |
|---|---|
| |

| |
| IUPAC名 Iron(II) phosphate | |
| 别名 | 磷酸铁(II) |
| 识别 | |
| CAS号 | 14940-41-1 |
| PubChem | 9863567 |
| ChemSpider | 8039263 |
| SMILES |
|
| 性质 | |
| 化学式 | Fe3O8P2 |
| 摩尔质量 | 357.48 g·mol−1 |
| 外观 | 无色固体[1] 蓝灰色晶体(八水)[2] |
| 密度 | 3.94 g/cm3[3] 2.58 g/cm3(八水合物)[4] |
| 熔点 | 180 °C(453 K)((八水合物)分解[6]) |
| 溶解性(水) | 不溶(八水)[5] |
| 溶解性 | 不溶于乙醇,可溶于酸(八水)[5] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
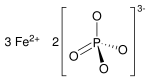
磷酸亚铁是磷酸的铁盐,化学式Fe3(PO4)2。它在自然界中以蓝铁矿形式存在[7],可用作肥料。[8]
制备
[编辑]- 3 FeSO4 + 2 Na3PO4 → Fe3(PO4)2↓ + 3 Na2SO4
磷酸和铁直接反应只会得到Fe(H2PO4)2·2H2O[1]。无水磷酸亚铁可以由硝酸铁和磷酸二氢铵反应,然后加热产生的棕色物质而成。[7]
物理性质
[编辑]无水磷酸亚铁呈单斜晶系,空间群P21/c (No. 14)。[3]其四水合物和八水合物的晶体结构也是单斜晶系,空间群分别为P21/a (No. 14)和C2/m (No. 12)。[9][10][11]
刚沉淀产生的八水合磷酸亚铁是蓝灰色固体,在空气氧化下转变成靛蓝色。对其进一步氧化会破坏其晶体结构,产生黄棕色的无定形体。[8]
化学性质
[编辑]- 水合物加热分解:
磷酸亚铁可以和N2H4形成各种配合物,如深绿色的Fe3(PO4)2·3N2H4·5H2O[12]、白色的Fe3(PO4)2·6N2H4·2.75H2O、[13]浅绿色的Fe3(PO4)2·6N2H4·8H2O[14]以及白色的Fe3(PO4)2·7N2H4·nH2O。[12]它们都会爆炸。
参见
[编辑]参考
[编辑]- ^ 1.0 1.1 1.2 Eisenphosphate Lexikon der Chemie. [2022-11-22]. (原始内容存档于2022-11-22).
- ^ CRC Handbook of Chemistry and Physics, 95 ed. CRC Press, 2014
- ^ 3.0 3.1 Kostiner, E.; Rea, J. R. Crystal structure of ferrous phosphate, Fe3(PO4)2. Inorganic Chemistry (American Chemical Society (ACS)). 1974, 13 (12): 2876–2880. ISSN 0020-1669. doi:10.1021/ic50142a021.
- ^ Jan D'Ans; Ellen Lax; Blachnik, Roger. Taschenbuch für Chemiker und Physiker : Band III: Elemente, anorganische Verbindungen und Materialien, Minerale. Berlin, Heidelberg. 2013: 458. ISBN 978-3-642-58842-6. OCLC 913685219 (德语).
- ^ 5.0 5.1 Hunnius, Curt; Burger, Artur. Hunnius pharmazeutisches Wörterbuch. Berlin. 2020: 469. ISBN 978-3-11-086901-9. OCLC 1138499573 (德语).
- ^ iron(II) phosphate octahydrate. chemister.ru. [2 July 2014]. (原始内容存档于2014-05-03).
- ^ 7.0 7.1 Warner, Joanne K.; Cheetham, Anthony K.; Nord, Anders G.; Von Dreele, Robert B.; Yethiraj, Mohana. Magnetic structure of iron(II) phosphate, sarcopside, Fe3(PO4)2. Journal of Materials Chemistry (Royal Society of Chemistry (RSC)). 1992, 2 (2): 191. ISSN 0959-9428. doi:10.1039/jm9920200191.
- ^ 8.0 8.1 Eynard, A.; Campillo, M. C.; Barrón, V.; Torrent, J. Use of vivianite (Fe3(PO4)2.8H2O) to prevent iron chlorosis in calcareous soils. Fertilizer Research (Springer Science and Business Media LLC). 1992, 31 (1): 61–67. ISSN 0167-1731. doi:10.1007/bf01064228.
- ^ Abrahams, S. C.; Bernstein, J. L. Crystal Structure of Paramagnetic Ludlamite, Fe3(PO4)2·4H2O, at 298°K. The Journal of Chemical Physics (AIP Publishing). 1966-03-15, 44 (6): 2223–2229. ISSN 0021-9606. doi:10.1063/1.1727026.
- ^ Meijer, H.C.; Van den Handel, J.; Frikkee, E. Magnetic behaviour of vivianite, Fe3(PO4)2.8H2O. Physica (Elsevier BV). 1967, 34 (3): 475–483. ISSN 0031-8914. doi:10.1016/0031-8914(67)90015-8.
- ^ Bartl, H. Water of crystallization and its hydrogen-bonded crosslinking in vivianite Fe3(PO4)2 · 8H2O; a neutron diffraction investigation. Fresenius' Zeitschrift für analytische Chemie (Springer Science and Business Media LLC). 1989, 333 (4-5): 401–403. ISSN 0016-1152. doi:10.1007/bf00572335.
- ^ 12.0 12.1 British Library. Lending Division, Royal Society of Chemistry (Great Britain), Chemical Society (Great Britain), Akademii︠a︡ nauk SSSR. Russian Journal of Inorganic Chemistry, Volume 32, Pages 919-1821. British Library Lending Division with the cooperation of the Royal Society of Chemistry. 1987: 973 [2022-11-22]. (原始内容存档于2022-11-22).
- ^ British Library. Lending Division, Royal Society of Chemistry (Great Britain), Chemical Society (Great Britain), Akademii︠a︡ nauk SSSR. Russian Journal of Inorganic Chemistry, Volume 27, Pages 1-913. British Library Lending Division with the cooperation of the Royal Society of Chemistry. 1982: 831 [2022-11-22]. (原始内容存档于2022-11-22).
- ^ British Library. Lending Division, Royal Society of Chemistry (Great Britain), Chemical Society (Great Britain), Akademii︠a︡ nauk SSSR. Russian Journal of Inorganic Chemistry, Volume 32, Pages 919-1821. British Library Lending Division with the cooperation of the Royal Society of Chemistry. 1987: 974 [2022-11-22]. (原始内容存档于2022-11-22).
延伸阅读
[编辑]- Редкол.: Кнунянц И.Л. и др. (编). Химическая энциклопедия. 2. М.: Советская энциклопедия. 1990: 671. ISBN 5-82270-035-5.
- Редкол.: Никольский Б.П. и др. (编). Справочник химика. 1 2-е изд., испр. М.-Л.: Химия. 1966: 1072.
- Редкол.: Никольский Б.П. и др. (编). Справочник химика. 2 3-е изд., испр. Л.: Химия. 1971: 1168.
- Рипан Р., Четяну И. Неорганическая химия. Химия металлов. 2. М.: Мир. 1972: 871.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.



![{\displaystyle {\mathsf {Fe_{3}(PO_{4})_{2}\cdot 8H_{2}O\ {\xrightarrow[{-H_{2}O}]{T))\ Fe_{3}(PO_{4})_{2}\cdot 4H_{2}O\ {\xrightarrow[{-H_{2}O}]{180^{o}C))\ Fe_{3}(PO_{4})_{2))))](https://wikimedia.org/api/rest_v1/media/math/render/svg/530df214323b0dc99e2019e7d43914c2b620eba9)
