三羰基环丁二烯合铁
| 三羰基环丁二烯合铁 | |
|---|---|

| |
| 识别 | |
| CAS号 | 12078-17-0 |
| 性质 | |
| 化学式 | (C4H4)Fe(CO)3 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
三羰基环丁二烯合铁,系统命名为三羰基·环丁二烯合铁(0)(化学式:(C4H4)Fe(CO)3)是一种中心铁原子与环丁二烯和羰基形成的有机金属化合物。这种特殊的配合物是有机化学中制备环丁二烯的主要前体之一。
制备
它最早由罗兰·佩蒂特(Rowland Pettit)[1] [2] [3]在1965年以环辛四烯为原料成功制备[註 1]:
环辛四烯氯化并重排生成7,8-二氯双环[4.2.0]-2,4-辛二烯,后者继续与含碳碳三键的丁炔二酸二甲酯发生Diels-Alder反应,然后在200°C下热解,通过逆向Diels-Alder反应得到顺式二氯环丁烯。
性质
三羰基环丁二烯合铁表现出芳香性,因为它参与的一些反应可归属于芳香亲电取代反应[4]:
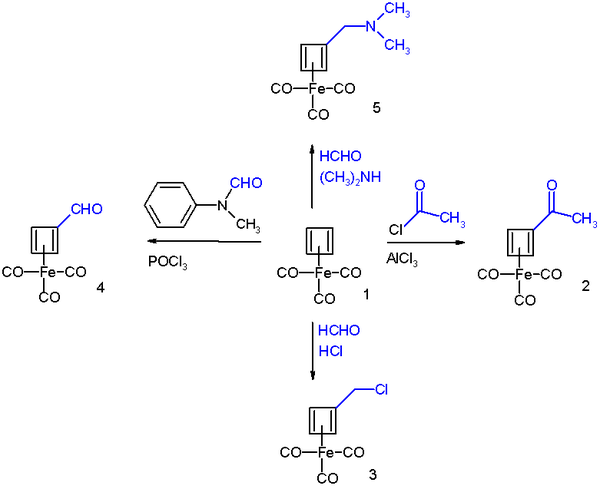
在Friedel–Crafts酰基化反应中,三羰基环丁二烯合铁与乙酰氯在三氯化铝催化下反应生成乙酰基衍生物2;与甲醛和盐酸发生氯甲基化反应生成氯甲基衍生物3;在Vilsmeier-Haack反应中,它与N-甲基甲酰苯胺和三氯氧磷反应得到甲酰基衍生物4;而它的Mannich反应可制得胺的衍生物4。
这些反应的反应机理与芳香亲电取代反应是相同的:
应用
三羰基环丁二烯合铁还是一种有机合成原料,例如它与二溴苯醌一起被硝酸铈铵氧化,再进行光化学反应形成基本骨架,再经过Favorskii重排反应形成四元环,最后脱羧得到立方烷[5][6]:
注:右下方物质少画了一根键
施密特和格拉布斯分别独立研究了这个反应,以确定该分子间环加成反应过程是产生了游离的环丁二烯还是以金属配合物进行反应。他们都认为该反应经历了一个非手性的中间体,因此只能得到外消旋的产物。后来朱利叶斯·里贝克的三相测试证实了环丁二烯中间体的存在。
三羰基环丁二烯合铁能够释放出活泼的中间体,经过很少的几步操作就可以生成所需的环系。一些问题还处于研究之中,例如该化合物作为双烯体或亲双烯体的反应,以及环丁二烯二聚机理的竞争等等。[7]
注释
- ^ reflux,3hrs.=回流3小时
参考资料
- ^ Cyclobutadiene- and Benzocyclobutadiene-Iron Tricarbonyl Complexes G. F. Emerson, L. Watts, R. Pettit; J. Am. Chem. Soc.; 1965; 87(1); 131-133. First Page (页面存档备份,存于互联网档案馆)
- ^ Cis-dichlorocyclobutene , Organic Syntheses, Coll. Vol. 6, p.422 (1988); Vol. 50, p.36 (1970) Article (页面存档备份,存于互联网档案馆).
- ^ Iron, tricarbonyl (η4-1,3-cyclobutadiene)- R. Pettit and J. Henery Organic Syntheses, Coll. Vol. 6, p.310 (1988); Vol. 50, p.21 (1970) Link (页面存档备份,存于互联网档案馆)
- ^ Cyclobutadieneiron Tricarbonyl. A New Aromatic System J. D. Fitzpatrick, L. Watts, G. F. Emerson, R. Pettit J. Am. Chem. Soc.; 1965; 87(14); 3254-3255 Abstract (页面存档备份,存于互联网档案馆)
- ^ Schmidt, E.K.G. Angew. Chem. Int. Ed. Engl. 1973, 12, P777-778
- ^ Grubbs, R.H.; Grey, R. A. J. Am. Chem. Soc. 1973, 95, P5765-5767
- ^ Tyler W. Wilson, Cyclobutadieneiron Tricarbonyl:synthetic Access To An Anti-aromatic System And Its Use In Synthesis, 2005
扩展阅读
- P. D. Harvey, W. P. Schaefer, H. B. Gray, D. F. R. Gilson, I. S. Butler. Structure of tricarbonyl(η4-cyclobutadienyl)iron(0) at −45 °C. Inorg. Chem. 1988, 27 (1): 57–59. doi:10.1021/ic00274a013.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.