硫氰
| 硫氰 | |
|---|---|
| |

| |
| |
| 识别 | |
| CAS号 | 505-14-6 |
| PubChem | 68160 |
| ChemSpider | 61468 |
| SMILES |
|
| InChI |
|
| InChIKey | DTMHTVJOHYTUHE-UHFFFAOYAE |
| ChEBI | 30063 |
| 性质 | |
| 化学式 | C2N2S2 |
| 摩尔质量 | 116.16 g·mol⁻¹ |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
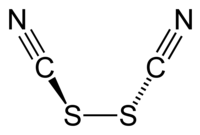
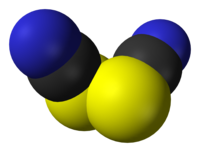
硫氰化學式為(SCN)2,是一种黄色液体,在常温下可以分解,是一种拟卤素。硫氰的空間對稱群為C2,其結構可表示為NCS-SCN[1]。硫氰的氧化能力比溴要大,會和水反應;[2]
- 3 (SCN)2 + 4 H2O → H2SO4 + HCN + 5 SCN− + 5 H+
硫氰最早是由硫氰酸银与碘在乙醚中制得[3],但由於碘的氧化性弱,此反應最後會達到平衡,產率不佳。硫氰也可以由硫氰酸鉛和Br2反应产生,而硫氰酸鉛可以由硝酸鉛和硫氰酸鈉混合而成。可以用無水的硫氰酸鉛和溴在冰醋酸中反應[4]。另一種方法是將溶於二氯甲烷中的溴滴入懸浮在0℃二氯甲烷的硫氰酸鉛中,之後再用氬氣保護,會生成硫氰的溶液,但製備的硫氰需立刻使用[5]。
- Pb(SCN)2 + Br2 → (SCN)2 + PbBr2
硫氰會和烯類產生加成反應,生成有1,2-二(硫氰)結構的化合物。硫氰也會和二茂钛杂环戊二烯(titanacyclopentadiene)反應,最後生成1,2-二噻烯(1,2-dithiins)。硒氰化學式為(SeCN)2,可以由硒氰酸银和碘在0度的四氢呋喃中反應制得[6],其反應和硫氰類似[5]。另外,硫氰能与硝酸反应,产生氢氰酸,一氧化氮,硫酸等;或被彻底氧化成CO2,NO2,SO3等气体。 制备硫氰也可以用硫氰化钾与碘发生置换反应,产生硫氰。
參考資料
[编辑]- ^ Jensen, James. Vibrational frequencies and structural determination of thiocyanogen. Journal of Molecular Structure: THEOCHEM. 2005, 714 (2–3): 137–141. doi:10.1016/j.theochem.2004.09.046.
- ^ Stedman, G.; Whincup, P. A. E. Oxidation of metal thiocyanates by nitric and nitrous acids. Part I. Products. Journal of the Chemical Society A: Inorganic, Physical, Theoretical. 1969: 1145. ISSN 0022-4944. doi:10.1039/j19690001145.
- ^ Söderbäck, Erik. Studien über das freie Rhodan. Justus Liebig's Annalen der Chemie. 1919, 419 (3): 217–322 [2023-03-14]. doi:10.1002/jlac.19194190302. hdl:2027/uc1.$b133351. (原始内容存档于2023-02-01).
- ^ Gardner, William Howlett; Weinberger, Harold. Inorganic Syntheses. Inorganic Syntheses. Inorganic Syntheses. 1939, 1: 84–86. ISBN 978-0-470-13232-6. doi:10.1002/9780470132326.ch29.
|chapter=被忽略 (帮助) - ^ 5.0 5.1 Block, E; Birringer, M; DeOrazio, R; Fabian, J; Glass, RS; Guo, C; He, C; Lorance, E; Qian, Q; Schroeder, TB; Shan, Z; Thiruvazhi, M; Wilson, GC; Zhang, Z. Synthesis, Properties, Oxidation, and Electrochemistry of 1,2-Dichalcogenins. J. Amer. Chem. Soc. 2000, 122: 5052–5064. doi:10.1021/ja994134s.
- ^ Meinke, PT; Krafft, GA; Guram, A. Synthesis of selenocyanates via cyanoselenation of organocopper reagents. J. Org. Chem. 1988, 53: 3632–3634. doi:10.1021/jo00250a047.
| ||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
