氯化镧
| 氯化镧 | |||
|---|---|---|---|
| |||

| |||
| 别名 | 三氯化镧 | ||
| 识别 | |||
| CAS号 | 10099-58-8 10025-84-0(七水) 17272-45-6(六水) 20211-76-1(水合) | ||
| PubChem | 64735 | ||
| ChemSpider | 58275 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | ICAKDTKJOYSXGC-DFZHHIFOAJ | ||
| 性质 | |||
| 化学式 | LaCl3 | ||
| 摩尔质量 | 245.26 g/mol (无水) 371.37 g/mol (七水) g·mol⁻¹ | ||
| 外观 | 白色无味潮解粉末 | ||
| 密度 | 3.84 g/cm3[1] | ||
| 熔点 | 858 °C (无水)[1] | ||
| 沸点 | 1000 °C (无水) | ||
| 溶解性(水) | 957 g·L−1(25 °C)[1] | ||
| 溶解性 | 七水物可溶于乙醇 | ||
| 结构 | |||
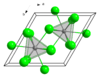
| 晶体结构 | 六方晶系三氯化铀型结构,hP8 | ||
| 空间群 | P63/m, No. 176 | ||
| 配位几何 | 三帽三角棱柱体 (九配位) | ||
| 危险性 | |||
| 欧盟编号 | 未列出 | ||
| 相关物质 | |||
| 其他阴离子 | 氟化镧 溴化镧 碘化镧 | ||
| 其他阳离子 | 氯化钪 氯化钇 氯化锕 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
氯化镧是一种无机化合物,化学式为LaCl3,它存在无水物和多种水合物,为白色粉末或无色晶体。
制备
[编辑]以从稀土矿物中得到的稀土氯化物或其硫酸铵复盐为原料,用氢氧化钠处理,并将其中的铈用过氧化氢氧化为四价,用稀盐酸浸出,得到富镧母液和富铈渣。富镧母液再经萃取分离除去铈后,经结晶得到氯化镧的水合物。[2]
- La(OH)3 + 3 HCl → LaCl3 + 3 H2O
将得到的氯化镧水合物在干燥的氯化氢中加热,可以得到无水物。[3]
它的无水物也可由氯化铵法制得,第一步反应形成五氯合镧酸铵,第二步将生成的铵盐在真空中于350~400 °C热分解:[4][5][6]
- La2O3 + 10 NH4Cl → 2 (NH4)2LaCl5 + 6 H2O + 6 NH3
- (NH4)2LaCl5 → LaCl3 + 2 HCl + 2 NH3
- La2O3 + 3 CCl4 → 2 LaCl3 + 3 COCl2
反应
[编辑]氯化镧和氟硅酸钠在水溶液中反应,生成氟化镧。[8]它和钽酸镧反应在高温反应,可以得到La2TaO4Cl3。[9]
应用
[编辑]氯化镧可以用作催化剂,如它可以催化邻苯二胺和苯甲醛的反应,得到2-苯基苯并咪唑;[10]它也可以催化甲烷的氯化反应。[11]
参考资料
[编辑]- ^ 1.0 1.1 1.2 Lide, David R. (编), CRC Handbook of Chemistry and Physics 87th, Boca Raton, FL: CRC Press, 2006, ISBN 0-8493-0487-3
- ^ 廖春生, 王嵩龄, 吴声. 氟碳铈矿制备富镧氯化稀土的方法 (页面存档备份,存于互联网档案馆). 2010. CN101914679 B
- ^ Observations on the Rare Earths. XXIX. The Preparation and Properties of Some Anhydrous Rare Earth Chlorides. J. Am. Chem. Soc. 1928, 50 (4): 959–967 [2023-09-01]. doi:10.1021/ja01391a005. (原始内容存档于2023-09-01).
- ^ Brauer, G. (编). Handbook of Preparative Inorganic Chemistry 2nd. New York: Academic Press. 1963.
- ^ Meyer, G. The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides-The Example of YCl3. Inorganic Syntheses 25. 1989: 146–150. ISBN 978-0-470-13256-2. doi:10.1002/9780470132562.ch35.
- ^ Edelmann, F. T.; Poremba, P. Herrmann, W. A. , 编. Synthetic Methods of Organometallic and Inorganic Chemistry VI. Stuttgart: Georg Thieme Verlag. 1997. ISBN 978-3-13-103021-4.
- ^ Gabbe, D. R., Folweiler, R. C., & Pink, F. X. Synthesis, Ultrapurification and Analysis of LaF3 for Fluoride Optical Fibers (88). MRS Proceedings. 1986 [2023-09-01]. doi:10.1557/proc-88-119. (原始内容存档于2023-09-01).
- ^ Bleshinskii, S. V.; Kharakoz, A. E.; Lukin, I. N.; Chalova, E. P. Reaction of hexafluorosilicates with salts of rare earth elements (俄文). Issled. Khim. Redk. Soputstv. Elem. 1966, (21-9).. CODEN 16CJAW
- ^ Schaffrath, U.; Gruehn, R. La2TaO4Cl3- a new oxochlorotantalate with TaO5 building units (德文). Zeitschrift fuer Naturforschung, B: Chemical Sciences: 412–418. ISSN 0932-0776.
- ^ Venkateswarlu, Y., Kumar, S.R. & Leelavathi, P. Facile and efficient one-pot synthesis of benzimidazoles using lanthanum chloride. Organic and Medicinal Chemistry Letters. 2013, 7 (3) [2023-09-01]. doi:10.1186/2191-2858-3-7. (原始内容存档于2023-09-01).
- ^ Elvira Peringer, Chirag Tejuja, Michael Salzinger, Angeliki A. Lemonidou, Johannes A. Lercher. On the synthesis of LaCl3 catalysts for oxidative chlorination of methane. Applied Catalysis A: General. 2008, 350 (2): 178–185. doi:10.1016/j.apcata.2008.08.009.
- ^ C Rosales, E J Brown. Calcium channel blockers nifedipine and diltiazem inhibit Ca2+ release from intracellular stores in neutrophils. Journal of Biological Chemistry. 1992, 267 (3): 1443–1448 [2023-09-01]. doi:10.1016/S0021-9258(18)45965-0. (原始内容存档于2023-09-01).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.