氯化钇
| 氯化钇 | |
|---|---|
| |
| IUPAC名 氯化钇(III) Yttrium(III) chloride | |
| 识别 | |
| CAS号 | 10361-92-9 |
| ChemSpider | 59696 |
| SMILES |
|
| InChI |
|
| InChIKey | PCMOZDDGXKIOLL-DFZHHIFOAW |
| RTECS | ZG3150000 |
| 性质 | |
| 化学式 | YCl3 |
| 摩尔质量 | 195.26 g·mol⁻¹ |
| 外观 | 白色固体 |
| 密度 | 2.67 g/cm3 |
| 熔点 | 721 °C |
| 沸点 | 1507 °C[1] |
| 溶解性(水) | 82 g/100 mL |
| 溶解性 | 60.1 g/100 mL乙醇 (15°C) 60.6 g/100 mL吡啶 (15°C)[2] |
| 结构 | |
| 晶体结构 | Monoclinic, mS16 |
| 空间群 | C12/m1, No. 12 |
| 危险性 | |
| 欧盟编号 | 未列出 |
| 闪点 | 不可燃 |
| 相关物质 | |
| 其他阴离子 | 氧化钇 |
| 其他阳离子 | 氯化钪 氯化镧 氯化锕 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
氯化钇是一种无机化合物,化学式为YCl3,易溶于水。
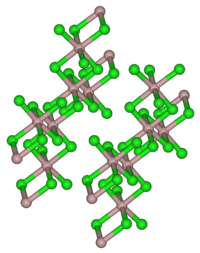
结构
[编辑]固体氯化钇有和AlCl3一样的结构。
制备和反应
[编辑]无水氯化钇通常由氯化铵和水合氯化钇、氧化钇、或氯氧化钇反应[3][4][5]首先得到(NH4)2[YCl5]:
- 10 NH4Cl + Y2O3 → 2 (NH4)2[YCl5] + 6 NH3 + 3 H2O
- YCl3·6H2O + 2 NH4Cl → (NH4)2[YCl5] + 6 H2O
然后将(NH4)2[YCl5]加热,使之分解:
- (NH4)2[YCl5] → 2 NH4Cl + YCl3
在分解的过程中,会产生中间体(NH4)[Y2Cl7]。
如果用盐酸作用于Y2O3,只会得到水合物(YCl3·6H2O),它不能通过直接加热得到无水物,因为在加热的过程中会水解,产生氯氧化钇。
参考资料
[编辑]- ^ Yttrium & Compounds, United States Occupational Safety and Health Administration, 2007-01-11 [2008-05-29], (原始内容存档于2013-03-02)
- ^ Spencer, James F., The Metals of the Rare Earths, New York: Longmans, Green, and Co: 135, 1919 [2008-05-29]
- ^ , Meyer, G. The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides-The Example of YCl3. Inorganic Syntheses. 1989, 25: 146–150. ISBN 978-0-470-13256-2. doi:10.1002/9780470132562.ch35.
- ^ Edelmann, F. T.; Poremba, P. Herrmann, W. A. (ed.) , 编. Synthetic Methods of Organometallic and Inorganic Chemistry VI. Stuttgart: Georg Thieme Verlag. 1997. ISBN 3-13-103021-6.
- ^ Taylor, M.D.; Carter, C.P. Preparation of anhydrous lanthanide halides, especially iodides. Journal of Inorganic and Nuclear Chemistry: 387–391. doi:10.1016/0022-1902(62)80034-7.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
