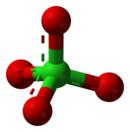
高氯酸盐
| 高氯酸盐 | |||
|---|---|---|---|

| |||
| |||
| 系统名 Perchlorate[1] | |||
| 识别 | |||
| CAS号 | 14797-73-0 | ||
| PubChem | 123351 | ||
| ChemSpider | 109953 | ||
| SMILES |
| ||
| Gmelin | 2136 | ||
| ChEBI | 49706 | ||
| DrugBank | DB03138 | ||
| MeSH | 180053 | ||
| IUPHAR配体 | 4524 | ||
| 性质 | |||
| 化学式 | ClO− 4 | ||
| 摩尔质量 | 99.451 g mol−1 g·mol⁻¹ | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
高氯酸盐是高氯酸形成的盐类,含有四面体型的高氯酸根离子—,其中氯的氧化态为+7。高氯酸盐存在于自然界中,主要用作火箭燃料和烟火中的氧化剂和安全气囊中的爆炸物。多数高氯酸盐可溶于水。[2]
常见的高氯酸盐有:
氧化性
[编辑]尽管其中氯的氧化态为+7,但在氯的所有含氧酸根离子中,高氯酸根离子在水溶液的氧化性最弱。[3]这是由于氯在高氯酸根离子中处于四面体的中心,不易发生反应。大多数高氯酸盐,尤其是电正性金属如钠形成的化合物如高氯酸钠,都相对较稳定,也因此可以取代曾经用于烟火中但具不稳定性的氯酸盐类氧化剂。[来源请求]
在溶液中,Ru(II)可以将还原为,V(II)、V(III)、Mo(III)、二聚Mo(III)、Cr(II)和Ti(III)都可将还原为。[4]
溶解性
[编辑]几乎所有高氯酸盐都可溶于水,且具有吸湿性或潮解性(铵盐、钾盐、铅盐和亚汞盐不潮解),高氯酸钾难溶于乙醇(和高氯酸钠不同)。[5]
参见
[编辑]参考文献
[编辑]- ^ Perchlorate - PubChem Public Chemical Database. The PubChem Project. USA: National Center for Biotechnology Information. [2019-04-07]. (原始内容存档于2014-04-27).
- ^ Draft Toxicological Profile for Perchlorates (页面存档备份,存于互联网档案馆), Agency for Toxic Substances and Disease Registry, U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES, September, 2005.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey, Advanced Inorganic Chemistry 5th, New York: Wiley-Interscience: 564, 1988, ISBN 0-471-84997-9
- ^ Edward T. Urbansky. Perchlorate Chemistry: Implications for Analysis and Remediation (页面存档备份,存于互联网档案馆), 1998
- ^ Prescott, A.B.; Johnson, O.C. Qualitative Chemical Analysis: A Guide in Qualitative Work, with Data for Analytical Operations and Laboratory Methods in Inorganic Chemistry. D. Van Nostrand Company. 1901: 342 [2021-11-27]. (原始内容存档于2021-11-27).
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.