磷的硫化物
磷的硫化物是一类无机化合物,只由磷和硫组成,通式为 P4Sx(x ≤ 10)。其中两种磷的硫化物具有商业意义:十硫化四磷 (P4S10)的产量达到千吨规模,用于生产有机硫化合物;而三硫化四磷 (P4S3)可用于制造火柴。
除了 P4S3 和P4S10以外,还存在多种磷的硫化物。其中六种有异构体:P4S4、P4S5、P4S6、P4S7、P4S8和 P4S9。它们的异构体以希腊字母前缀区别。这些前缀是以发现顺序排列的,而不是结构。[1] 所有已知的磷的硫化物分子的四个磷原子都为四面体型排列(类似白磷)。[2] P4S2 也是已知的,不过在 −30 °C以上不稳定。[3]
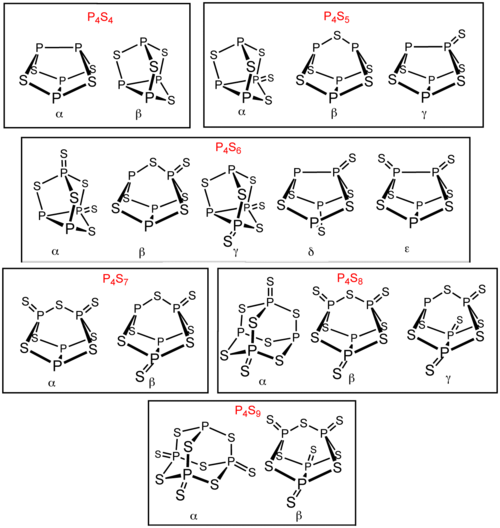
制备
[编辑]磷的硫化物的主要制备方法是加热磷和硫的混合物。产物可以由 31P 核磁共振波谱法分析。更具选择性的合成需要用三苯基膦脱硫,或是用硫化三苯基胂硫化。[4][5]
P4S3
[编辑]三硫化四磷可以由红磷和硫在 450 K以上反应,[6] 然后用二硫化碳和苯小心地重结晶。另一种制备方法是将白磷和硫在惰性、不易燃的溶剂里反应而成。[7]
P4S4
[编辑]α型和β型的 P4S4可以由 P4S3I2 的对应异构体和((CH3)3Sn)2S 反应而成:[6]
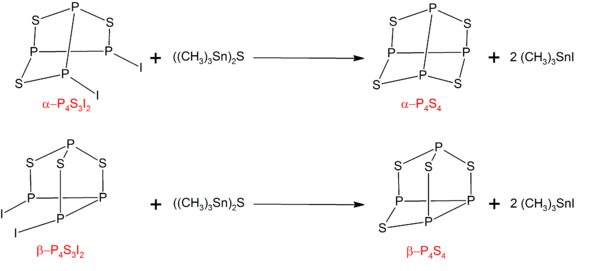
反应前体 P4S3I2 则可以由化学计量的磷、硫和碘反应而成。
P4S5
[编辑]P4S5 可以由化学计量的 P4S3 和硫的二硫化碳溶液在光照和催化量的碘下反应而成。[8]产物可以通过使用磷-31核磁共振波谱法分析。
α-P4S5 可以通过 P4S10和红磷的光化学反应而成。[6] P4S5 是对热不稳定的,会在达到熔点之前歧化成 P4S3 和 P4S7。[9]
P4S6
[编辑]P4S6 可以由 P4S7 被三苯基膦夺走一个硫原子而成:[6]
α-P4S5 和溶于二硫化碳的Ph3AsS 反应,也可以得到 α-P4S6。[4]两种新的六硫化四磷异构体δ-P4S6 和ε-P4S6可以由 α-P4S4 和溶于二硫化碳的Ph3SbS 反应而成。[10]
P4S7
[编辑]P4S7 可以由磷和硫直接反应而成,是最容易提纯的磷的硫化物之一。[11]
- 4 P + 7 S → P4S7
P4S8
[编辑]β-P4S8可以由 α-P4S7 和溶于二硫化碳的 Ph3AsS 反应而成,会产生α-P4S7 和β-P4S8的混合物。[4]
P4S9
[编辑]P4S9 的制备有两种方法。一种方法是 P4S3和过量的硫加热反应而成。[6]
另一种方法则是以1:2 的比例混合P4S7 和 P4S10,会产生 P4S9。这个反应为可逆反应:[10]
- P4S7 + 2 P4S10 ⇌ 3 P4S9
P4S10
[编辑]P4S10是最稳定的磷的硫化物之一。它可以通过在真空管中,将白磷和硫加热到 570 K 以上而成。[12]
- P4 + 10 S → P4S10
参考资料
[编辑]- ^ Jason, M. E.; Ngo, T.; Rahman, S. Products and Mechanisms in the Oxidation of Phosphorus by Sulfur at Low Temperature. Inorg. Chem. 1997, 36 (12): 2633–2640. doi:10.1021/ic9614879.
- ^ Holleman, A. F.; Wiberg, E. Inorganic Chemistry. Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Heal, H. G. The Inorganic Heterocyclic Chemistry of Sulfur, Nitrogen, and Phosphorus Academic Press: London; 1980 ISBN 0-12-335680-6.
- ^ 4.0 4.1 4.2 Jason, M. E. Transfer of Sulfur from Arsenic and Antimony Sulfides to Phosphorus Sulfides. Rational Syntheses of Several Less-Common P4Sn Species. Inorg. Chem. 1997, 36 (12): 2641–2646. doi:10.1021/ic9614881.
- ^ Nowottnick, H.; Blachnik, R. Zwei neue Phosphorsulfide (Two New Phosphorus Sulfides). Zeitschrift für anorganische und allgemeine Chemie. 1999, 625 (12): 1966–1968. doi:10.1002/(SICI)1521-3749(199912)625:12<1966::AID-ZAAC1966>3.0.CO;2-B.
- ^ 6.0 6.1 6.2 6.3 6.4 Catherine E. Housecroft; Alan G. Sharpe. Chapter 15: The group 15 elements. Inorganic Chemistry, 3rd Edition. Pearson. 2008: 484. ISBN 978-0-13-175553-6.
- ^ "Phosphorus trisulfide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 563.
- ^ "Phosphorus pentasulfide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 565.
- ^ A. Earnshaw; Norman Greenwood. Phosphorus. Chemistry of the elements, 2nd edition. Butterworth Heinemann. 2002: 508. ISBN 0750633654.
- ^ 10.0 10.1 R. Bruce King. Phosphorus. Encyclopedia of Inorganic Chemistry, 2nd edition. Wiley. 2005: 3711. ISBN 9780470862100.
- ^ "Phosphorus heptasulfide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 566.
- ^ "Diphosphorus pentasulfide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 567.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
