亚硒酸
| 亚硒酸 | |
|---|---|
| |

| |

| |
| 英文名 | Selenous acid |
| 识别 | |
| CAS号 | 7783-00-8 |
| PubChem | 1091 |
| ChemSpider | 1060 |
| SMILES |
|
| InChI |
|
| InChIKey | MCAHWIHFGHIESP-UHFFFAOYAW |
| ChEBI | 26642 |
| KEGG | D05814 |
| 性质 | |
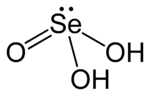
| 化学式 | H 2SeO 3 |
| 摩尔质量 | 128.979 g·mol⁻¹ |
| 外观 | 无色潮解性晶体 |
| pKa | 2.46, 7.3[1] |
| 相关物质 | |
| 其他阴离子 | 硒酸 硫酸 亚硫酸 |
| 其他阳离子 | 亚硒酸钠 |
| 相关化学品 | 二氧化硒 三氧化硒 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
亚硒酸(化学式:H2SeO3,或写作(HO)2SeO),是硒的含氧酸的一种,其中硒的氧化态为+4。它是白色正交晶系晶体,极易溶于水,由二氧化硒溶于少量水缓慢蒸發结晶并用氢氧化钾干燥得到。晶体中稍许畸变的SeO3基团,靠较强的氢键相互连接。
亚硒酸是二元中强酸,中和程度不同时会分别得到亚硒酸氢盐(含HSeO3−)和亚硒酸盐(含SeO32−):[2]
- H2SeO3 ⇌ H+ + HSeO3− pKa = 2.62
- HSeO3− ⇌ H+ + SeO32− pKa = 8.32
浓度超过4mol/L的亚硒酸会发生二聚生成H2Se2O5,并脱去一分子水。它与亚硫酸不同,溶液不游离出二氧化硒,固态亚硒酸加热至150°C分解为二氧化硒。
亚硒酸是一个中强的氧化剂,动力学上不活泼。酸性溶液中,它可以氧化二氧化硫、硫化氢、硫脲、碘化钾、硫代硫酸钠、氨、肼、羟胺等还原性物质,半反应为:
- H2SeO3 + 4H+ + 4e− ⇌ Se + 3H2O ; E
o= +0.74 V
碱性溶液中:
- SeO32− + 4e− + 3H2O ⇌ Se + 6OH− ; E
o= −0.37 V
它在有机合成中用于制取1,2-二羰基化合物(如乙二醛)。[3]
在更强的氧化剂(如臭氧、過氧化氫、高锰酸根离子)作用下,亚硒酸也可以被氧化为硒酸。亚硒酸有很高毒性,中毒症状可能延迟数小时,包括昏迷、恶心、低血压,严重时可能致死。[4]
参见
[编辑]参考资料
[编辑]- ^ Ka and pKa for Polyprotic Acids. ucdsb.on.ca
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ “Glyoxal Bisulfite” (页面存档备份,存于互联网档案馆), Organic Syntheses, Collected Volume 3, p.438 (1955).
- ^ MSDS for "Reagent for Special Opiates (Codeine, Heroin, & Morphine)" (页面存档备份,存于互联网档案馆), Sirchie Finger Print Laboratories, Inc. May 12, 2006.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
