穴状配体
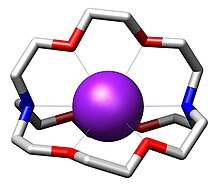
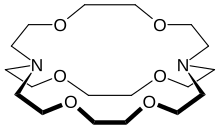
穴醚是一类人工合成的,可以与阳离子发生配位的双环和多环多齿配体。[2]“穴醚(cryptand)”一词是指该配体形如空穴,将底物分子容纳在里面。整个分子是一个三维的结构。因此与单环的冠醚相比,穴醚配合物更加稳定,对底物分子的选择性也更强。形成的复合物具有脂溶性。唐纳德·克拉姆、让-马里·莱恩和查尔斯·佩特森通过对穴醚和冠醚进行研究,开创了超分子化学的先例,并因此获得了1987年的诺贝尔化学奖。[3]
结构
[编辑]最为常见且最为重要的穴醚是N[CH2CH2OCH2CH2OCH2CH2]3N(右图),IUPAC名称为1,10-二氮杂-4,7,13,16,21,24-六氧杂双环[8.8.8]二十六碳烷,俗称[2.2.2]-穴醚。方括号内的数字表示在两个氮桥头之间每个桥上的氧原子个数。
全胺穴醚对碱金属阳离子具有极高的亲和力,通过穴醚与碱金属作用,可以成功得到含K−等碱金属阴离子的盐类。[4]
性质
[编辑]穴醚的三维内部空腔可以和外来离子紧密结合,形成的复合物被称为穴状化合物(cryptate)。结合能力最强的是较硬的阳离子,包括NH4+(铵离子)和镧系元素、碱金属、碱土金属的阳离子。穴醚利用分子中的氮和氧与这些离子配位,由于不同的离子与不同三维结构的穴醚结合能力不同,通过选取适当的穴醚,可以将不同的碱金属阳离子区分或分离出来。
与冠醚类似,大环穴醚一般也是利用胺和卤代烃的缩合反应制备的。但由于环系更为复杂,穴醚的产率通常不高。
用途
[编辑]穴醚的制备较为困难,且价格昂贵;但是,与冠醚之类的其他配位剂相比,穴醚与碱金属离子结合更紧密,选择性更强。[5] 通过配位,穴醚可以将一般情况下不溶于有机溶剂的盐类溶于另一相中,用作相转移催化剂,加快化学反应的速率;[6] 也可以稳定碱金属负离子,使碱化物和电子盐得以合成。另外,穴醚还可以帮助Sn92−之类津特耳离子(Zintl ion)的结晶。
在製作核医学需要的顯像用藥物氟代脫氧葡萄糖(簡稱 FDG)時,會使用[2.2.2]穴醚來絡合反應物K18F中的鉀離子,提高放射性18F離子的親核性[7],以便將18F連接到脫氧葡萄糖中。
参阅
[编辑]参考文献
[编辑]- ^ Alberto, R.; Ortner, K.; Wheatley, N.; Schibli, R.; Schubiger, A. P. Synthesis and properties of boranocarbonate: a convenient in situ CO source for the aqueous preparation of [99mTc(OH2)3(CO)3]+. J. Am. Chem. Soc. 2001, 121: 3135–3136. doi:10.1021/ja003932b.
- ^ von Zelewsky, A. Stereochemistry of Coordination Compounds; John Wiley: Chichester, 1995. ISBN 0-471-95057-2.
- ^ Lehn, J. M. Supramolecular Chemistry: Concepts and Perspectives; VCH: Weinhiem, 1995.
- ^ Kim, J.; Ichimura, A. S.; Huang, R. H.; Redko, M.; Phillips, R. C.; Jackson, J. E.; Dye, J. L. Crystalline salts of Na− and K− (Alkalides) that are stable at room temperature. J. Am. Chem. Soc. 1999, 121: 10666–10667. doi:10.1021/ja992667v.
- ^ Dietrich, B. "Cryptands" in Comprehensive Supramolecular Chemistry; Gokel, G. W. Ed; Elsevier: Oxford, 1996; Vol. 1, pp 153–211. ISBN 0080406106.
- ^ Landini, D.; Maia, A.; Montanari, F.; Tundo, P. Lipophilic [2.2.2] cryptands as phase-transfer catalysts. Activation and nucleophilicity of anions in aqueous-organic two-phase systems and in organic solvents of low polarity. J. Am. Chem. Soc. 1979, 101: 2526–2530. doi:10.1021/ja00504a004.
- ^ 王榮; 伍洲; 李彥生; 姜申德. 正電子發射斷層顯像中的氟-18 標記藥物. THE CHINESE CHEM. SOC., TAIPEI. 2006, 64 (2): 179–200.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
