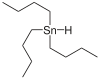
氢化三丁基锡
| 氢化三丁基锡 | |||
|---|---|---|---|
| |||

| |||
| 系统名 Tributylstannane[1] | |||
| 识别 | |||
| CAS号 | 688-73-3 | ||
| PubChem | 5948 | ||
| ChemSpider | 5734 | ||
| SMILES |
| ||
| Beilstein | 3587329 | ||
| Gmelin | 4258 | ||
| EINECS | 211-704-4 | ||
| ChEBI | 27086 | ||
| MeSH | Tributyltin | ||
| 性质 | |||
| 化学式 | SnC 12H 28 | ||
| 摩尔质量 | 291.06 g mol−1 g·mol⁻¹ | ||
| 密度 | 1.082 g cm−3 | ||
| 沸点 | 80 °C(353 K) | ||
| 溶解性(水) | 缓慢分解 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
氢化三丁基锡 是一种有机锡化合物 ,化学式 (C4H9)3SnH。 它是一种无色液体,可溶于有机溶剂。 这种化合物是有机合成里,氢原子的来源。
制备和特征
[编辑]氢化三丁基锡可以由氧化三丁基锡被聚甲基氢硅氧烷还原而成 (Bu = CH3CH2CH2CH2):[2][3]
- 2"(MeSiH)" + (Bu3Sn)2O → "(Me2Si)2O" + 2 Bu3SnH
- (Bu3Sn)2O + 2/n (MeSi(H)O)n → 2 Bu3SnH + 1/n [(MeSiO)2O]n
氢化物是一种可蒸馏的液体,对空气有轻微的敏感,会分解成(Bu3Sn)2O。 其红外光谱在νSn-H的1814cm-1处显示很强的能带。
应用
[编辑]它是有机合成里是一种有用的试剂。 它和偶氮二异丁腈 (AIBN) 混合或被光照时, 氢化三丁基锡会将有机卤化物(和相关基团)转化为相应的烃。 此过程是通过涉及自由基Bu3Sn•的自由基反应机制发生的。 [4][5] 该基团从另一个氢化三丁基锡分子中夺取H•,从而传播该链。 氢化三丁基锡作为H•供体的用途可归因于其相对较弱的键强(78 kcal/mol)。 [6]
它是氢锡基化反应的选择性试剂: [7]
- RC2R' + HSnBu3 → RC(H)=C(SnBu3)R'
参见
[编辑]扩展阅读
[编辑]- Hayashi, K.; Iyoda, J.; Shiihara, I. "Reaction of organotin oxides, alkoxides and acyloxides with organosilicon hydrides. New preparative method of organotin hydrides " J. Organomet. Chem. 1967, 10, 81. doi:10.1016/S0022-328X(00)81719-2
参考资料
[编辑]- ^ SnBu3H - PubChem Public Chemical Database. The PubChem Project. USA: National Center for Biotechnology Information. [2020-08-23]. (原始内容存档于2012-11-03).
- ^ Maleczka, Robert E.; Terrell, Lamont R.; Clark, Damon H.; Whitehead, Susan L.; Gallagher, William P.; Terstiege, Ina. Application of Fluoride-Catalyzed Silane Reductions of Tin Halides to the in Situ Preparation of Vinylstannanes. J. Org. Chem. 1999, 64: 5958–5965. doi:10.1021/jo990491+.
- ^ Tormo, J.; Fu, G. C. α-D-Ribo-hexofuranose, 3-deoxy-1,2:5,6-bis-O-(1-methylethylidene). Org. Synth. 2002, 78: 239. doi:10.15227/orgsyn.078.0239.
- ^ OUP catalogue page (页面存档备份,存于互联网档案馆), J. Clayden, N. Greeves, S. Warren and P. Wothers, in Organic Chemistry, 2000, OUP, Oxford, ch. 39, pp. 1040-1041.
- ^ T. V. (Babu) RajanBabu, Philip C. Bulman Page, Benjamin R. Buckley, "Tri-n-butylstannane" Encyclopedia of Reagents for Organic Synthesis 2004, John Wiley & Sons. doi:10.1002/047084289X.rt181.pub2
- ^ Laarhoven, L. J. J.; Mulder, P.; Wayner, D.D. M. "Determination of Bond Dissociation Enthalpies in Solution by Photoacoustic Calorimetry" Acc. Chem. Res. 1999, 32, 342 doi:10.1021/ar9703443
- ^ Smith, Nicholas D.; Mancuso, John; Lautens, Mark. Metal-Catalyzed Hydrostannations. Chemical Reviews. 2000, 100 (8): 3257–3282. PMID 11749320. doi:10.1021/cr9902695.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.