二碲化钨
| 二碲化钨[1] | |
|---|---|

| |
| |
| 英文名 | Tungsten(IV) telluride |
| 别名 | 碲化钨(IV) |
| 识别 | |
| CAS号 | 12067-76-4 |
| PubChem | 82913 |
| SMILES |
|
| InChI |
|
| InChIKey | WFGOJOJMWHVMAP-UHFFFAOYSA-N |
| EINECS | 235-086-0 |
| 性质 | |
| 化学式 | WTe2 |
| 摩尔质量 | 439.04 g·mol⁻¹ |
| 外观 | 灰色晶体[2] |
| 密度 | 9.43 g/cm3,固体 |
| 熔点 | 972 °C(1245 K)(分解[3]) |
| 溶解性(水) | 不溶 |
| 溶解性 | 不溶于氨,盐酸和硫酸[3],在硝酸里分解[3] |
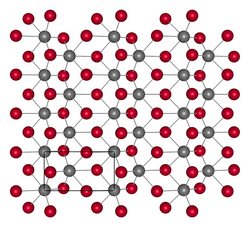
| 结构[4] | |
| 晶体结构 | 正交晶系,oP12 |
| 空间群 | Pmn21, No. 31 |
| 晶格常数 | a = 3.50 Å, b = 6.34 Å, c = 15.4 Å |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
制备
[编辑]二碲化钨可以由钨和碲在 800 °C 的真空下直接反应而成。[5]
性质
[编辑]二碲化钨是一种灰色固体,[2]不溶于水。在 650 °C 时,二碲化钨会和氧气反应,600 °C时在真空中分解。[3]它是正交晶系的,空间群 Pmn21 (No. 31)。[6] 它有着层状结构。[7]
用处
[编辑]二碲化钨可用于半导体工业。[8]
参考资料
[编辑]- ^ Lide, David R. Handbook of Chemistry and Physics 87. Boca Raton, Florida: CRC Press. 1998: 4–92. ISBN 0-8493-0594-2.
- ^ 2.0 2.1 William M. Haynes, [《二碲化钨》在Google Books的內容。 CRC Handbook of Chemistry and Physics, 94th Edition], CRC Press. 2016: pp. 97, (德文)
- ^ 3.0 3.1 3.2 3.3 Erik Lassner, Wolf-Dieter Schubert, [《二碲化钨》在Google Books的內容。 Tungsten Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds], Springer Science & Business Media. 2012: pp. 167, (德文)
- ^ Persson, Kristin. Materials Data on Te2W by Materials Project. LBNL Materials Project; Lawrence Berkeley National Laboratory (LBNL), Berkeley, CA (United States). 2020. OSTI 1198898. doi:10.17188/1198898.
- ^ Jane E. Callanan, G.A. Hope, Ron D. Weir, Edgar F. Westrum: Thermodynamic properties of tungsten ditelluride (WTe2) I. The preparation and lowtemperature heat capacity at temperatures from 6 K to 326 K. In: The Journal of Chemical Thermodynamics. 24, 1992, S. 627, doi:10.1016/S0021-9614(05)80034-5.
- ^ B. E. Brown: The crystal structures of WTe2 and high-temperature MoTe2. In: Acta Crystallographica. 20, 1966, S. 268, doi:10.1107/S0365110X66000513.
- ^ Chia-Hui Lee, Eduardo Cruz Silva, Lazaro Calderin, Minh An T. Nguyen, Matthew J. Hollander, Brian Bersch, Thomas E. Mallouk, Joshua A. Robinson: Tungsten Ditelluride: a layered semimetal. In: Scientific Reports. 5, 2015, S. 10013, doi:10.1038/srep10013.
- ^ Dale L. Perry, [《二碲化钨》在Google Books的內容。 Handbook of Inorganic Compounds, Second Edition], CRC Press. 2016: pp. 442, (德文)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

