δ軌域

在化學與原子物理學中,δ軌域(英語:δ orbital, delta orbital)是一種分子軌域。是形成δ鍵後所產生的分子軌域。δ軌域是一種由d軌域四重交疊而成所形成的新軌域。
δ軌域會出現在已經形成δ鍵的化合物中,而已經形成δ鍵的化合物多半是有機金屬化合物中,尤其是釕、鉬和錸所形成的化合物,因此,這些化合物中皆可以找到δ軌域。
除了δ軌域外,還有一些軌域也是面對面重疊後所形成的分子軌域,例如:φ軌域、γ軌域。
結構
[编辑]
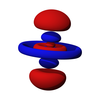
δ軌域是一種由軌域面對面重疊後所形成的分子軌域,主要是由d軌域所形成,較常是由兩個原子的dxy軌域或 dx2-y2軌域發生交互作用而形成。由於這些分子軌域涉及低能量d軌域,他們被視為過渡金屬配合物。
δ軌域的電子出現概率在原子附近與d軌域相同,然後離原子較遠的地方開始減少,直到δ鍵正中央時降為0,然後到靠近另外一個原子之處又升高,在另外一個原子支出的電子出現概率形狀也與d軌域相同,中間電子出現概率降為0之處稱為波節面,除了兩原子中間有波節面外,每個葉中也可以有波節。
δ鍵
[编辑]參考文獻
[编辑]- 曾國輝《原子結構》建宏出版社 台北市 1999 ISBN 957-724-801-2
- 曾國輝《化學鍵》建宏出版社 台北市 1999 ISBN 957-724-802-0
參見
[编辑]
| |||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.