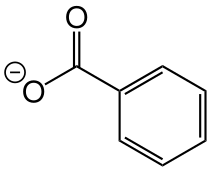
苯甲酸镍
| 苯甲酸镍 | ||
|---|---|---|
|
| ||
| 识别 | ||
| CAS号 | 553-71-9 | |
| PubChem | 3083628 | |
| SMILES |
| |
| InChI |
| |
| 性质 | ||
| 化学式 | (C6H5COO)2Ni | |
| 摩尔质量 | 300.927(无水) 354.975(三水) g·mol⁻¹ | |
| 外观 | 亮绿色针状晶体[1] | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | ||
苯甲酸镍是Ni2+的苯甲酸盐,化学式为(C6H5COO)2Ni。
制备
[编辑]- 2 C6H5COOH + Ni(OH)2 + H2O → (C6H5COO)2Ni·3H2O
三水合物在142~252℃失去一分子结晶水,在252~318℃变为无水物。
以镍片作为阳极,铂片作为阴极,电解苯甲酸的丙酮溶液,可以制备无水苯甲酸镍。[3]
化学性质
[编辑]苯甲酸镍受热可以分解,在氮气气氛中,分解产物是碳酸镍和一些有机化合物,碳酸镍可以进一步分解为一氧化镍和二氧化碳。分解出的有机物主要有三苯甲烷和二苯甲酮;联苯、芴等也被检测到。[2]
三水合苯甲酸镍在真空中,除了失水外,还会产生苯甲酸、苯酚、碳、镍、氧化镍、碳化镍等产物。[4]
另有文献指出,苯甲酸镍的无水物和水合物有着不同的稳定性及热分解过程。无水物可以稳定至230℃,受热的最终产物是一氧化镍;而四水合物在100℃便开始分解,受热的最终产物是金属镍。[1]
配合物
[编辑]苯甲酸镍可以形成[Ni2(μ2-Bz)4(HBz)2](Bz=C6H5COO−)双核配合物,它可通过苯甲酸钠、硝酸镍、2,2'-联吡啶的溶剂热反应制备。[1]
苯甲酸镍也可以和联氨形成配合物Ni(C6H5COO)2(N2H4)2,为蓝色晶体,可以通过硝酸镍和苯甲酸联氨在水溶液中反应得到。[5]
参考文献
[编辑]- ^ 1.0 1.1 1.2 Anna Vráblová, Larry R. Falvello, Javier Campo, et al. Preparation, First Structure Analysis, and Magnetism of the Long-Known Nickel Benzoate Trihydrate - A Linear Ni…Ni…Ni Polymer and Its Parallels with the Active Site of Urease. Eur. J. Inorg. Chem., 2016(6): 928–934. doi: 10.1002/ejic.201501255
- ^ 2.0 2.1 宋力, 赵艳茹, 袁良杰 等. 苯甲酸镍的流变相法合成及热分解机理研究. 化学试剂, 2004. 26(3): 129-131, 139
- ^ 陈胜洲, 鲁德平, 杨世芳. 电化学法合成无水苯甲酸镍的研究. 化学与生物工程, 2001. (2):11-12
- ^ AK Galwey. The thermal decomposition of nickel benzoate and of the nickel salt of cyclohexanecarboxylic acid. J. Chem. Soc., 1965, 6188-6194 DOI: 10.1039/JR9650006188
- ^ K. Kuppusamy, S. Govindarajan. Benzoate Complexes of Dipositive First Row Transition Metal Ions with Hydrazine. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 1995. pp 225-243. DOI: 10.1080/00945719608004260.
| |||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.