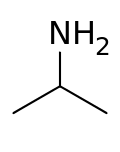
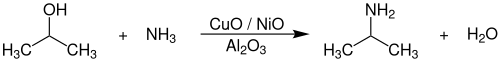异丙胺
| 异丙胺 | |||
|---|---|---|---|
| |||
| IUPAC名 Propan-2-amine 2-丙胺 | |||
| 别名 |
| ||
| 识别 | |||
| CAS号 | 75-31-0 | ||
| PubChem | 6363 | ||
| ChemSpider | 6123 | ||
| SMILES |
| ||
| Beilstein | 605259 | ||
| 3DMet | B01040 | ||
| UN编号 | 1221 | ||
| EINECS | 200-860-9 | ||
| ChEBI | 15739 | ||
| RTECS | NT8400000 | ||
| KEGG | C06748 | ||
| MeSH | 2-propylamine | ||
| 性质 | |||
| 化学式 | C3H9N | ||
| 摩尔质量 | 59.11 g·mol−1 | ||
| 外观 | 无色液体 | ||
| 气味 | 氨味 | ||
| 密度 | 0.694 g·cm−3(15 °C) | ||
| 熔点 | -101 °C(172 K) | ||
| 沸点 | 32 °C(305 K) | ||
| 溶解性(水) | 混溶 | ||
| log P | 0.391 | ||
| 蒸气压 | 63.41 kPa(20 °C) | ||
| 折光度n D |
1.3742 | ||
| 热力学 | |||
| ΔfHm⦵298K | −113.0–−111.6 kJ mol−1 | ||
| ΔcHm⦵ | −2.3540–−2.3550 MJ mol−1 | ||
| S⦵298K | 218.32 J K−1 mol−1 | ||
| 热容 | 163.85 J K−1 mol−1 | ||
| 危险性 | |||
GHS危险性符号 
| |||
| GHS提示词 | 危险 | ||
| H-术语 | H224, H315, H319, H335 | ||
| P-术语 | P210, P261, P305+351+338 | ||
| 爆炸极限 | 2–10.4% | ||
| PEL | TWA 5 ppm (12 mg/m3)[1] | ||
| 致死量或浓度: | |||
LD50(中位剂量)
|
| ||
LC50(中位浓度)
|
4,000 ppm (大鼠, 4 h)[2] | ||
LCLo(最低)
|
7000 ppm (小鼠, 40 min)[2] | ||
| 相关物质 | |||
| 相关化学品 | 正丙胺 异丙醇 2-硝基丙烷 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
异丙胺(简写IPA、MIPA)是一种有机化合物,化学式为C3H9N,它是具有氨味的无色吸湿性液体,可燃。它是重要的化工中间体。[3]
合成
异丙胺可由异丙醇和氨气在氢气的存在下,以氧化铝负载的氧化铜、氧化镍催化剂催化,在180~220 °C、30~60 bar下反应得到:[4]
性质
异丙胺可以和醛发生缩合反应,如和苯甲醛反应,生成N-亚苄基异丙胺;[6]它也能和酰氯反应,如和联苯-4,4'-二甲酰氯在三乙胺的存在下反应,生成N,N '-二异丙基联苯-4,4'-二甲酰胺。[7]
- C3H9N + HNO3 → [C3H9NH]NO3
- C3H9N + HCOOH → [C3H9NH]HCOO
参考文献
- ^ NIOSH Pocket Guide to Chemical Hazards. #0360. NIOSH.. 2019-10-30. [2021-09-09]
- ^ 2.0 2.1 Isopropylamine. Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health. 4 December 2014 [14 April 2015]. (原始内容存档于2021-09-09) (英语).
- ^ Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut, Amines, Aliphatic, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, 2000-06-15, doi:10.1002/14356007.a02_001 (英语)
- ^ Asprion, Norbert; Bey, Oliver; Huyghe, Kevin; Julius, Manfred; Kummer, Matthias; Melder, Johann-Peter; Moors, Maarten; Mägerlein, Wolfgang; Stein, Frank; Werland, Stefanie. Method for Producing Ethylamines and Monoisopropylamine (MIPA). EP2782898B1. [2021-09-09]. (原始内容存档于2021-09-09) (德语).
- ^ Zhou, Junjie; Li, Yunong; Sun, Hong-bin; Tang, Zhike; Qi, Li; Liu, Lei; Ai, Yongjian; Li, Shuang; Shao, Zixing; Liang, Qionglin. Porous silica-encapsulated and magnetically recoverable Rh NPs: a highly efficient, stable and green catalyst for catalytic transfer hydrogenation with “slow-release” of stoichiometric hydrazine in water. Green Chemistry (Royal Society of Chemistry (RSC)). 2017, 19 (14): 3400–3407. ISSN 1463-9262. doi:10.1039/c7gc00986k (英语).
- ^ Talotta, Carmen; Concilio, Gerardo; De Rosa, Margherita; Soriente, Annunziata; Gaeta, Carmine; Rescifina, Antonio; Ballester, Pablo; Neri, Placido. Expanding Coefficient: A Parameter To Assess the Stability of Induced-Fit Complexes. Organic Letters (American Chemical Society (ACS)). 2021-02-16, 23 (5): 1804–1808. ISSN 1523-7060. doi:10.1021/acs.orglett.1c00165 (英语).
- ^ Maiti, Avijit; Chandra, Shubhadeep; Sarkar, Biprajit; Jana, Anukul. Acyclic diaminocarbene-based Thiele, Chichibabin, and Müller hydrocarbons. Chemical Science (Royal Society of Chemistry (RSC)). 2020, 11 (43): 11827–11833. ISSN 2041-6520. doi:10.1039/d0sc03622f (英语).
- ^ Reynolds, John L.; Erdner, Kimberly R.; Jones, Paul B. Photoreduction of Benzophenones by Amines in Room-Temperature Ionic Liquids. Organic Letters (American Chemical Society (ACS)). 2002, 4 (6): 917–919. ISSN 1523-7060. doi:10.1021/ol017290o (英语).
- ^ Collins, Matthew P.; Zhou, Ling; Camp, Suzanne E.; Danielson, Neil D. Isopropylammonium Formate as a Mobile Phase Modifier for Liquid Chromatography. Journal of Chromatographic Science (Oxford University Press (OUP)). 2012-06-19, 50 (10): 869–876. ISSN 1945-239X. doi:10.1093/chromsci/bms084 (英语).
外部链接
- 国际化学品安全卡0908
- NIOSH Pocket Guide to Chemical Hazards. #0360. NIOSH.
| |||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.