仲丁胺
| 仲丁胺 | |
|---|---|
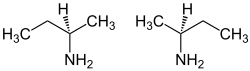
| |
| IUPAC名 Butan-2-amine | |
| 别名 |
|
| 识别 | |
| 缩写 | 2-AB |
| CAS号 | 13952-84-6 13250-12-9((R)) 513-49-5((S)) |
| PubChem | 24874 2724537((R)) 6713753((S)) |
| ChemSpider | 23255, 2006669 (R), 5145745 (S) |
| SMILES |
|
| Beilstein | 1361345, 1718761 (R), 1718760 (S) |
| UN编号 | 2733 |
| EINECS | 237-732-7 |
| ChEBI | 74526 |
| RTECS | EO3325000 |
| KEGG | C18706 |
| 性质 | |
| 化学式 | C4H11N |
| 摩尔质量 | 73.14 g·mol−1 |
| 外观 | 无色液体 |
| 气味 | 鱼腥,类似氨 |
| 密度 | 0.724 g cm−3 |
| 熔点 | -105 °C(168.65 K) |
| 沸点 | 63 °C(336 K) |
| 溶解性(水) | 互溶[1] |
| 折光度n D |
1.3928 |
| 黏度 | 500 μPa s (at 20 °C) |
| 热力学 | |
| ΔfHm⦵298K | −138.5 to −136.5 kJ mol−1 |
| ΔcHm⦵ | −3.0095 to −3.0077 MJ mol−1 |
| 危险性 | |
GHS危险性符号   
| |
| GHS提示词 | DANGER |
| H-术语 | H225, H302, H314, H332, H400 |
| P-术语 | P210, P273, P280, P305+351+338, P310 |
| NFPA 704 | |
| 致死量或浓度: | |
LD50(中位剂量)
|
|
| 相关物质 | |
| 相关烷基胺 | |
| 相关化学品 | 2-甲基-2-亚硝基丙烷 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
仲丁胺是一种有机化合物(更确切地,一种胺),分子式为CH3CH2CH(NH2)CH3。它是一种无色液体。仲丁胺是丁烷的四个异构胺之一,其他是正丁胺、叔丁胺和异丁胺。仲丁胺具有手性,因此可以以两种对映异构体之一的形式存在。
仲丁胺用于一些杀虫剂的生产中。[2]
安全
参考文献
- ^ ICSC 0401 - sec-BUTYLAMINE. [2022-01-15]. (原始内容存档于2022-04-23).
- ^ 2.0 2.1 Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut, Amines, Aliphatic, Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, 2005, doi:10.1002/14356007.a02_001
- ^ United States Environmental Protection Agency.
| |||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.


