二苯基二硒醚
| 二苯基二硒醚 | |
|---|---|

| |

| |
| IUPAC名 1,1′-Diselanediyldibenzene | |
| 别名 | 二苯基二硒 |
| 识别 | |
| CAS号 | 1666-13-3 |
| PubChem | 15460 |
| ChemSpider | 14710 |
| SMILES |
|
| InChI |
|
| InChIKey | YWWZCHLUQSHMCL-UHFFFAOYAK |
| RTECS | JM9152500 |
| 性质 | |
| 化学式 | C12H10Se2 |
| 摩尔质量 | 312.13 g·mol−1 |
| 外观 | 黄色晶体 |
| 密度 | 1.84 g/cm3 |
| 熔点 | 59-61 °C(332-334 K) |
| 溶解性(水) | 不溶 |
| 溶解性 | 可溶于二氯甲烷、THF和热己烷 |
| 结构 | |
| 偶极矩 | 0 D |
| 危险性 | |
| 警示术语 | R:R23/25 R33 R50/53 |
| 安全术语 | S:S20/21 S28 S45 S60 S61 |
| 主要危害 | 有毒 |
| 相关物质 | |
| 相关化学品 | Ph2S2 C6H5SeH |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
二苯基二硒醚是一种有机硒化合物,化学式 (C6H5)2Se2,简称Ph2Se2。这种橙色固体是苯硒酚的氧化产物。在有机合成中,它用来引入 PhSe基团。
制备和性质
Ph2Se2 可以由苯硒酚盐(含有 PhSe-的化合物)的氧化而成,后者则可以由格氏试剂和硒反应而成:[1]
这种分子的对称性为 C2,这类似于过氧化氢。Se-Se 键长为 2.29 Å ,C-Se-Se-C 二面角为 82°,C-Se-Se 键角接近 110°。[2]
反应
Ph2Se2 可以被还原:
- Ph2Se2 + 2 Na → 2 PhSeNa
PhSeNa 是一种有用的亲核试剂,可以对卤代烷烃、磺酸酯(甲磺酸酯或对甲苯磺酸酯)和环氧化物进行亲核取代反应。它在制备吗啡的例子中出现:[3]
它也可以被氯化:
- Ph2Se2 + Cl2 → 2 PhSeCl
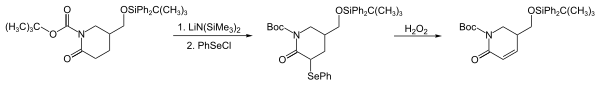
PhSeCl 是一种强大的亲电试剂,可以和各种亲电试剂反应,包括烯醇盐、烯醇硅醚、格氏试剂、有机锂化合物、烯烃和胺,并引入 PhSe基团。在以下反应中(马钱子酚的早期合成步骤),通过内酰胺烯醇盐与 PhSeCl 的反应中引入了 PhSe 基团。 [4]这种反应可以把羰基化合物转化成对应的 α,β-不饱和化合物。[5]
二苯基二硒醚本身也是弱亲电性的 PhSe基团来源,会和强亲核体,像是格氏试剂和锂试剂反应。PhSeCl 的反应性更强,效率也更高,因为使用 Ph2Se2 会浪费掉一半的硒。
- Ph2Se2 + Nu− → PhSeNu + PhSe−
当 PhSeCl太强而二苯基二硒醚太弱时,N-苯基硒代邻苯二甲酰亚胺 (N-PSP) 也可以用来取代 PhSeCl。[6]
参考资料
- ^ Reich, H. J. (1979). "Reagents for Synthesis of Organoselenium Compounds: Diphenyl Diselenide and Benzeneselenenyl Chloride". Org. Synth. 59: 141; Coll. Vol. 6: 533.
- ^ Marsh, R. E. The Crystal Structure of Diphenyl Diselenide. Acta Crystallographica. 1952, 5 (4): 458–462. doi:10.1107/S0365110X52001349
 .
.
- ^ Taber, D. F.; Neubert, T. D.; Rheingold, A. L. Synthesis of (−)-Morphine. Journal of the American Chemical Society. 2002, 124 (42): 12416–12417. PMID 12381175. doi:10.1021/ja027882h.
- ^ Lerchner, A.; Carreira, E. M. First Total Synthesis of (±)-Strychnofoline via a Highly Selective Ring-Expansion Reaction. Journal of the American Chemical Society. 2002, 124 (50): 14826–14827. PMID 12475306. doi:10.1021/ja027906k.
- ^ Reich, H. J.; Wollowitz, S. Preparation of α,β-Unsaturated Carbonyl Compounds and Nitriles by Selenoxide Elimination. Organic Reactions. 1993, 44: 1–296. ISBN 0471264180. doi:10.1002/0471264180.or044.01.
- ^ Barrero, A. F.; Alvarez-Manzaneda, E. J.; Chahboun, R.; Corttés, M.; Armstrong, V. Synthesis and Antitumor Activity of Puupehedione and Related Compounds. Tetrahedron. 1999, 55 (52): 15181–15208. doi:10.1016/S0040-4020(99)00992-8.
| ||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.