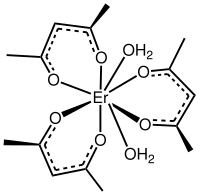
乙酰丙酮铒
| 乙酰丙酮铒 | |
|---|---|
| |
| 识别 | |
| CAS号 | 14553-08-3(无水) 16788-89-9(三水) |
| 性质 | |
| 化学式 | C15H21ErO6 |
| 摩尔质量 | 464.58 g·mol−1 |
| 外观 | 粉色晶体[1] |
| 熔点 | 103 °C(376 K)[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
乙酰丙酮铒是一种配位化合物,化学式为Er(C5H7O2)3,或简写为Er(acac)3。它可由金属铒[2]或三氢化铒[1]和乙酰丙酮反应制得。氯化铒和乙酰丙酮铵反应,也能得到乙酰丙酮铒,它可以在甲苯中重结晶。[3]其无水物在干燥的气氛中稳定,在潮湿空气中形成水合物。[4]在潮湿气氛中加热水合物不能得到无水物。[5]它在190 °C开始分解,在505 °C持续加热可以得到氧化铒。[3]
参考文献
- ^ 1.0 1.1 1.2 Janice M. Koehler, William G. Bos. A novel synthesis of some anhydrous rare earth acetylacetonates. Inorganic and Nuclear Chemistry Letters. 1967-12, 3 (12): 545–548 [2021-09-20]. doi:10.1016/0020-1650(67)80023-0 (英语).
- ^ J.R. Blackborow, C.R. Eady, E.A.Koerner Von Gustorf, A. Scrivanti, O. Wolfbeis. Chemical syntheses with metal atoms. Journal of Organometallic Chemistry. 1976-03, 108 (3): C32–C34 [2021-09-20]. doi:10.1016/S0022-328X(00)92025-4. (原始内容存档于2018-06-26) (英语).
- ^ 3.0 3.1 J. Blanusa, B. Antic, A. Kremenovic, A.S. Nikolic, L. Mazzerolles, S. Mentus, V. Spasojevic. Particle size effect on Néel temperature in Er2O3 nanopowder synthesized by thermolysis of 2, 4-pentadione complex. Solid State Communications. 2007-11, 144 (7-8): 310–314 [2021-09-20]. doi:10.1016/j.ssc.2007.09.003. (原始内容存档于2018-06-16) (英语).
- ^ Trembovetskii, G. V.; Martynenko, L. I.; Murav'eva, I. A.; Spitsyn, V. Synthesis and study of volatile rare earth acetylacetonates. Doklady Akademii Nauk SSSR, 1984. 277 (6): 1411-1414. ISSN: 0002-3264. (俄文)
- ^ Martynenko, L. I.; Murav'eva, I. A.; Khalmurzaev, N. K.; Spitsyn, V. I. Preparation of volatile rare earth element tris(acetylacetonates) by the heating of their hydrates. Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1984. 6: 1207-1211. ISSN 0002-3353. (俄文)
| |||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
