2-Butanol
| |
| Nazivi | |
|---|---|
| IUPAC naziv
Butan-2-ol[1]
| |
| Drugi nazivi
sec-Butanol
sec-Butil alkohol 2-Butanol 2-Butil alkohol | |
| Identifikacija | |
| |
3D model (Jmol)
|
|
| Bajlštajn | 773649 1718764 (R) |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.053 |
| EC broj | 201-158-5 |
| Gmelin Referenca | 1686 396584 (R) |
| MeSH | 2-butanol |
| RTECS | EO1750000 |
| UN broj | 1120 |
| |
| Svojstva | |
| C4H10O | |
| Molarna masa | 74,12 g·mol−1 |
| Gustina | 0,808 g cm−3 |
| Tačka topljenja | −115 °C; −175 °F; 158 K |
| 290 g dm−3[4] | |
| log P | 0.683 |
| Napon pare | 1,67 kPa (na 20 °C) |
| Indeks refrakcije (nD) | 1,3978 (na 20 °C) |
| Termohemija | |
| Specifični toplotni kapacitet, C | 197,1 J K−1 mol−1 |
| Standardna molarna entropija S |
213,1 J K-1 mol−1 |
Std entalpija
formiranja (ΔfH⦵298) |
−343,3–−342,1 kJ mol-1 |
| Std entalpija sagorevanja ΔcH |
−2,6611–−2,6601 MJ mol−1 |
| Opasnosti | |
| Bezbednost prilikom rukovanja | inchem.org |
| GHS grafikoni |  
|
| GHS signalna reč | Upozorenje |
| H226, H319, H335, H336 | |
| P261, P305+351+338 | |
EU klasifikacija (DSD)
|
|
| R-oznake | R10, R36/37, R67 |
| S-oznake | (S2), S7/9, S13, S24/25, S26, S46 |
| NFPA 704 | |
| Tačka paljenja | 22 to 27 °C (72 to 81 °F; 295 to 300 K) |
| 405 °C (761 °F; 678 K) | |
| Eksplozivni limiti | 1.7–9.8% |
| Letalna doza ili koncentracija (LD, LC): | |
LCLo (LCLo)
|
16,000 ppm (pacov, 4 hr) 10,670 ppm (miš, 3.75 hr) 16,000 ppm (miš, 2.67 hr)[5] |
| SAD zdravstvene granice izlaganja (NIOSH): | |
PEL (dozvoljivo)
|
TWA 150 ppm (450 mg/m3)[5] |
REL (preporučeno)
|
TWA 100 ppm (305 mg/m3) ST 150 ppm (455 mg/m3)[5] |
IDLH (neposredna opasnost)
|
2000 ppm[5] |
| Srodna jedinjenja | |
Srodne butanoli
|
n-Butanol Izobutanol tert-Butanol |
Srodna jedinjenja
|
Butanon |
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25 °C [77 °F], 100 kPa). | |
| Reference infokutije | |
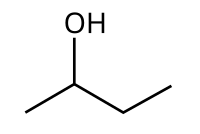
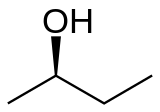
2-Butanol (sec-butanol) je organsko jedinjenje sa formulom CH3CH(OH)CH2CH3. Ovaj sekundarni alkohol je zapaljiva, bezbojna tečnost, koja je rastvorna u 12 delova vode i kompletno se meša sa polarnim organskim rastvaračima, kao što su etri i drugi alkoholi. On se proizvodi u velikim razmerama, prvenstveno kao prekurzor industrijskog rastvarača metil etil ketona. 2-Butanol je hiralan, te postoje dva stereoizomera, koji se označavaju kao (R)-(−)-2-butanol i (S)-(+)-2-butanol. On se normalno nalazi kao smeša dva stereoizomera – racemska smeša.
 |

|
 |

|
Proizvodnja i primene
[уреди | уреди извор]2-Butanol se industrijski proizvodi hidratacijom 1-butena ili 2-butena:
Sumporna kiselina se koristi kao katalizator over konverzije.[6]
Mada se deo proizvedenog 2-butanola koristi kao rastvarač, on se uglavnom konvertuje do butanona ("MEK"), koji je važan industrijski rastvarač i prisutan je u sredstvima za čišćenje i uklanjanje farbe. Isparljivi estri 2-butanola imaju prijatne arome, te se u malim količinama koriste u parfemima i veštačkim začinima.
Reference
[уреди | уреди извор]- ^ „2-butanol - Compound Summary”. PubChem Compound. USA: National Center for Biotechnology Information. 26. 3. 2005. Identification and Related Records. Приступљено 12. 10. 2011.
- ^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
- ^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
- ^ Alger, Donald B. (1991). „The water solubility of 2-butanol: A widespread error”. Journal of Chemical Education. USA: ACS Publications. 68 (11): 939. doi:10.1021/ed068p939.1. Приступљено 12. 10. 2011.
- ^ а б в г NIOSH Džepni vodič hemijskih hazarda 0077
- ^ Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2005.
Spoljašnje veze
[уреди | уреди извор]- Internacionalna karta hemijske bezbednosti 0112
- NIOSH Džepni vodič hemijskih hazarda 0077
- IPCS Ekološki zdravstveni kriterijum 65: Butanols: four isomers
| (0°) | |
|---|---|
| Primarni alkoholi (1°) | |
| Sekondarni alkoholi (2°) | |
| Tercijarni alkoholi (3°) | |
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.


