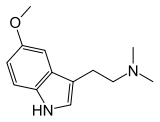
5-MeO-DMT
Izvor: Wikipedija
| |||
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| 2-(5-metoks-1H-indol-3-il)-N,N-dimetiletanamin | |||
| Klinički podaci | |||
| Identifikatori | |||
| CAS broj | 1019-45-0 | ||
| ATC kod | nije dodeljen | ||
| PubChem[1][2] | 1832 | ||
| ChemSpider[3] | 1766 | ||
| KEGG[4] | C08309 | ||
| ChEBI | CHEBI:2086 | ||
| ChEMBL[5] | CHEMBL7257 | ||
| Hemijski podaci | |||
| Formula | C13H18N2O | ||
| Mol. masa | 218,298 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | ? (UK) Plan I(SAD) | ||
| Način primene | pušenjem, uduvavanjem | ||
5-MeO-DMT (5-metoksi-N,N-dimetiltriptamin) je moćan psihodelični triptamin. On je prisutan u širokom nizu različitih biljki i psihoaktivnih žaba. On se poput njegovih bliskih srodnika DMT i bufotenina (5-HO-DMT) od davnina koristio u Južnoj Americi kao enteogen.
5-MeO-DMT je prvi put sintetisan 1936, i 1959. je izolovan kao jedan od psihoaktivnih sastojaka Anadenanthera peregrina semena. On se javlja u mnogim organizmima koji sadrže bufotenin (5-OH-DMT). On je O-metilni analog tog jedinjenje. 5-MeO-DMT se metaboliše prvenstveno posredstvom CYP2D6.[6]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Shen HW, Jiang XL, Winter JC, Yu AM. Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Current Drug Metabolism. 2010 Oct;11(8):659-66. PMID 20942780
| Aminokiseline | Alanin • Aspartat • Cikloserin • DMG • GABA • Glutamat • Glicin • Hipotaurin • Kinurenska kiselina (Transtorin) • NAAG (Spaglumska kiselina) • NMG (Sarkozin) • Serin • Taurin • TMG (Betain) |
|---|---|
| Endokanabinoidi | |
| Gasotransmiteri | |
| Monoamini | |
| Purini | |
| Trag amini | |
| Drugi | 1,4-BD • Acetilholin • GBL • GHB • Histamin |
Vidi isto Neuropeptidi | |
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
