正负离子半径比
正负离子半径比或阳阴离子半径比(英語:Cation-anion radius ratio)是在凝聚态物理和无机化学中用来预测离子化合物晶体结构的参数。其定义为离子化合中带正电的阳离子半径与带负电的阴离子半径的比值。通常情况下,阴离子的半径比阳离子半径大,因此常是阴离子占据晶格位点,阳离子则填充阴离子之间的空隙,因此可以根据几何关系预测晶体的结构。该法则是鲍林法则中的第一规则[1]。在给定结构下,可简称为半径比。
半径比规则与稳定性
[编辑]
半径比规则是指根据离子的配位方式的不同,每种配位方式存在一个临界的半径比[2]。其思想是将离子化合物中的阴阳离子视为不可压缩球体,意味着阴阳离子两种大小不同的“球”发生不等最密堆积,在给定结构下允许的阳离子大小范围由临界半径比决定[3]。如果阳离子太小,被吸引过来的阴离子之间会发生接触,由于同种电荷相互排斥,这种情况下其结构不稳定,也就是说当半径比下降到该特定结构的临界半径比以下时,就会发生这种情况。在刚好处于稳定极限时,阳离子接触所有阴离子,而阴离子只接触它们的边缘。对于大于临界半径比的半径比,结构预计是稳定的。
然而并非所有化合物都遵循这一规则。据估计,晶体结构只有约2/3的情况被这一规则猜中[4]。产生这种误差的原因是实际上化合物离子之间并非完全是离子键,有时会带有一定共价键成分[2]。
下表列出了临界半径比与配位数之间的关系,从简单的几何学关系,可以得到下式:[5]
| 临界半径比 | 配位数 | 间隙类型 | 晶体结构 | 示例 |
|---|---|---|---|---|
| 0.1547 | 3 |  平面三角 |
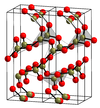 α-B 2O 3 结构 |
B2O3 |
| 0.2247 | 4 |  正四面体 |
 闪锌矿结构 |
ZnS, CuCl |
| 0.4142 | 6 | 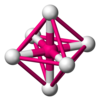 正八面体 |
 岩盐结构 |
NaCl, MgO |
| 0.7320 | 8 | 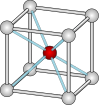 立方体 |
 氯化铯结构 |
CsCl, NH4Br |
历史
[编辑]正负离子半径比最初由Gustav F. Hüttig在1820年提出[6][7]。1926年戈尔德施密特将其推广到离子晶格中[8][9][10]。1929年,这一规则被合并到描述晶体结构的鲍林法则中,并作为当中的第一条规则[11]。
相关条目
[编辑]参考文献
[编辑]- ^ 梁敬魁. 相图与相结构. 北京: 科学出版社. 1993. ISBN 9787030031624.
- ^ 2.0 2.1 Michmerhuizen, Anna; Rose, Karine; Annankra, Wentiirim; Vander Griend, Douglas A. Radius Ratio Rule Rescue. Journal of Chemical Education (American Chemical Society (ACS)). 2017-08-09, 94 (10): 1480–1485. ISSN 0021-9584. doi:10.1021/acs.jchemed.6b00970.
- ^ Pauling, Linus. Nature of the Chemical Bond 3rd. Ithaca, New York: Cornell University Press. 1960: 544. ISBN 9780801403330.
- ^ Nathan, Lawrence C. Predictions of crystal structure based on radius ratio: How reliable are they?. Journal of Chemical Education (American Chemical Society (ACS)). 1985, 62 (3): 215. ISSN 0021-9584. doi:10.1021/ed062p215.
- ^ Toofan, Jahansooz. A Simple Expression between Critical Radius Ratio and Coordination Number. Journal of Chemical Education (American Chemical Society (ACS)). 1994, 71 (2): 147. ISSN 0021-9584. doi:10.1021/ed071p147. (and Erratum 71(9): 749 doi:10.1021/ed071p749), Following the erratum, equations should read and .
- ^ Jensen, William B. The Origin of the Ionic-Radius Ratio Rules. Journal of Chemical Education (American Chemical Society (ACS)). 2010-04-23, 87 (6): 587–588. ISSN 0021-9584. doi:10.1021/ed100258f.
- ^ Hüttig, Gustav F. Notiz zur Geometrie der Koordinationszahl. Zeitschrift für anorganische und allgemeine Chemie (Wiley). 1920-11-11, 114 (1): 24–26. ISSN 0863-1786. doi:10.1002/zaac.19201140103 (德语).
- ^ Goldschmidt, V.; Barth, T.; Lunde, G.; Zachariasen, W. Geochemische Verteilungsgesetze der Elemente. VII. Die Gesetze der Krystallochemie. Oslo: Dybwad. 1926: 112–117 [2024-07-09]. OCLC 174577644. (原始内容存档于2023-06-29) (德语).
- ^ Goldschmidt, V. Geochemische Verteilungsgesetze der Elemente. VIII. Untersuchungen über Bau und Eigenschaften von Krystallen. Oslo: Dybwad. 1927: 14–17. OCLC 19831825 (德语).
- ^ Goldschmidt, V. M. Crystal structure and chemical constitution. Transactions of the Faraday Society (Royal Society of Chemistry (RSC)). 1929, 25: 253. ISSN 0014-7672. doi:10.1039/tf9292500253.
- ^ Pauling, Linus. The principles determining the structure of complex ionic crystals. Journal of the American Chemical Society (American Chemical Society (ACS)). 1929, 51 (4): 1010–1026. ISSN 0002-7863. doi:10.1021/ja01379a006.
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.








