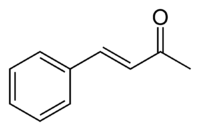
亚苄基丙酮
| 亚苄基丙酮 | |
|---|---|
| |
| |
| IUPAC名 4-Phenyl-3-buten-2-one | |
| 别名 | Benzalacetone Benzylideneacetone Methyl styryl ketone Benzylidene acetone |
| 识别 | |
| CAS号 | 122-57-6 1896-62-4(反式) |
| PubChem | 11147801(顺式) 637759(反式) |
| ChemSpider | 21106584 |
| SMILES |
|
| InChI |
|
| InChIKey | BWHOZHOGCMHOBV-BQYQJAHWBQ |
| ChEBI | 217301 |
| RTECS | EN0330000 |
| 性质 | |
| 化学式 | C10H10O |
| 摩尔质量 | 146.19 g/mol g·mol⁻¹ |
| 外观 | 黄白色固体 |
| 密度 | 1.008 g/cm3 |
| 熔点 | 39–42 °C |
| 沸点 | 260–262 °C |
| 溶解性(水) | 1.3 g/L |
| 溶解性(其他溶剂) | 非极性溶剂 |
| 危险性 | |
| 警示术语 | R:36/37/38-43 |
| 安全术语 | S:22-26-36/37 |
| 主要危害 | 刺激性 |
| 闪点 | 116 °C |
| 相关物质 | |
| 相关化学品 | 二亚苄基丙酮 肉桂醛 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
亚苄基丙酮是一种有机化合物,结构简式为C6H5CH=CHC(O)CH3。虽然从理论上该α,β-不饱和酮化合物同时存在顺反异构体,但至今只发现了其反式异构体。该化合物的最早合成方法说明了缩合反应可以合成诸多复杂的全新有机化合物。[1]
制备
[编辑]亚苄基丙酮可简便的通过氢氧化钠介导的丙酮与苯甲醛反应制备:[2]
反应
[编辑]如同大多数的甲基酮化合物,亚苄基丙酮是一种alpha位具有温和酸性的化合物,非常容易通过去质子化得到相应的烯醇化合物。[3]

该化合物因含有以下官能团而可发生相应反应,如:双键对溴素的加成反应;杂二烯烃化合物对于烯烃的Diels-Alder反应,得到二氢吡喃化合物;甲基可进一步与苯甲醛发生缩合反应得到二亚苄基丙酮;羰基可形成腙。该化合物还可与Fe2(CO)9反应得到(亚苄基丙酮)Fe(CO)3,这是一种可将Fe(CO)3传递给其他有机化学底物的试剂。[4]
参考文献
[编辑]- ^ Claisen, L. "Über die Einwirkung von Aceton auf Furfural und auf Benzaldehyd bei Gegenwart von Alkalilauge" Berichte der deutschen chemischen Gesellschaft 1881, volume 14, p 2468-2471.
- ^ Drake, N. L.; Allen, Jr. P.. "Benzalacetone". Org. Synth.; Coll. Vol. 1: 77.
- ^ Danheiser, R. L.; Miller, R. F.; Brisbois, R. G. (1990). "Detrifluoroacetylative Diazo Group Transfer: (E)-1-Diazo-4-phenyl-3-buten-2-one". Org. Synth. 73: 134; Coll. Vol. 9: 197.
- ^ Knölker, H.-J. "(η4-Benzylideneacetone)tricarbonyliron" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. Onlinedoi:10.1002/047084289X.rb058.
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

