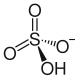
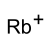硫酸氢铷
| 硫酸氢铷 | |||
|---|---|---|---|
| |||
| 识别 | |||
| CAS号 | 15587-72-1 | ||
| PubChem | 23689122 | ||
| ChemSpider | 141556 | ||
| SMILES |
| ||
| 性质 | |||
| 化学式 | RbHSO4 | ||
| 摩尔质量 | 182,54 g/mol−1 g·mol⁻¹ | ||
| 外观 | 无色晶体[1] | ||
| 密度 | 2.89 g·cm−3 | ||
| 熔点 | 214 °C(487 K) | ||
| 相关物质 | |||
| 其他阳离子 | 氧化铷 氢氧化铷 | ||
| 相关化学品 | 硫酸铷 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
制备
可以用水和等同化学计量的焦硫酸铷来合成硫酸氢铷。 反应发生在没有湿度的地方: [2]
还有另一种合成硫酸氢铷的方法。它类似于硫酸钠和硫酸钾的合成。 该反应需要氯化铷和少量的硫酸,反应期间还会产生一些氯化氢。
性质
它是一种可水解的化合物。 它是单斜晶系 的,结构为 P21/n。 硫酸氢铷的晶格参数为: a = 1440 pm、b = 462.2 pm、 c = 1436 pm 和 β = 118.0°。 它的晶体和硫酸氢铵同构。 [3]
其焓为-1166 kJ/mol。 [4] 在其溶解于水的过程中,产生了15.62 kJ/mol的能量。 [5]
加热时,硫酸氢铷会分解成焦硫酸铷和水:[6]
参考资料
- ^ Jean D'Ans, Ellen Lax: Taschenbuch für Chemiker und Physiker. 3. Elemente, anorganische Verbindungen und Materialien, Minerale, Band 3. 4. Auflage, Springer, 1997, ISBN 978-3-5406-0035-0, S. 692 (Taschenbuch für Chemiker und Physiker,第692页,载于Google图书).
- ^ S. B. Rasmussen, H. Hamma, K. M. Eriksen, G. Hatem, M. Gaune-Escard, R. Fehrmann: "Physico-chemical properties and transition metal complex formation in alkali pyrosulfate and hydrogen sulfate melts". VII International Conference on Molten Slags Fluxes and Salts, The South African Institute of Mining and Metallurgy, 2004. Volltext (页面存档备份,存于互联网档案馆) (PDF; 661 kB)
- ^ J. P. Ashmore, H. E. Petch: "The Structure of RbHSO4 in its Paraelectric Phase" in Can. J. Phys 1975, 53(24), S. 2694-2702. doi:10.1139/p75-328
- ^ L. A. Cowan, R. M. Morcos, N. Hatada, A. Navrotsky, S. M. Haile: "High temperature properties of Rb3H(SO4)2 at ambient pressure: Absence of a polymorphic, superprotonic transition" in Solid State Ionics 2008, 179, S. 305-313. Volltext (页面存档备份,存于互联网档案馆) (PDF; 837 kB)
- ^ M. de Forcrand: "Sur les chlorures et sulfates de rubidium et de caesium" in Compt. Rend. Hebd. 1906, 143, S. 98. Volltext (页面存档备份,存于互联网档案馆)
- ^ R. Abegg, F. Auerbach: "Handbuch der anorganischen Chemie". Verlag S. Hirzel, Bd. 2, 1908. S. 432.Volltext
| |||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.