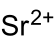溴酸锶
| 溴酸锶 | ||
|---|---|---|
|
| ||
| 英文名 | Strontium bromate | |
| 识别 | ||
| CAS号 | 14519-18-7 | |
| PubChem | 9819472 | |
| SMILES |
| |
| InChI |
| |
| EINECS | 238-531-7 | |
| 性质 | ||
| 化学式 | Br2O6Sr | |
| 摩尔质量 | 343.42 g·mol−1 | |
| 外观 | 无色到微黄色的潮解晶体[1] | |
| 密度 | 3.773 g·cm−3[1](一水) | |
| 熔点 | 240 °C(分解)[1] | |
| 溶解性 | 373 g·l−1 (25 °C)[1] | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | ||
溴酸锶,化学式Sr(BrO3)2,是实验室的或工厂中都很少考虑使用的化学品,但在奥利佛·萨克斯出版的《钨丝舅舅》提到过它。在那里是说:溴酸锶从饱和水溶液中析出结晶会变热。[2] 在化学上,这种盐可溶于水,而且是一种比较强的氧化剂。[3] 它对皮肤有刺激性,吸入或摄入它对身体有害。
制备
性质

溴酸锶易溶于水。1000 g 的水在 0 °C下能溶解183,2 g的溴酸锶,在25 °C下为272,5 g ,在 104 °C下为410 g。它通常以一水合物 Sr(BrO3)2 · H2O 的形式存在,水合物在 75.5 °C 下失水。[5]
一水合物是单斜晶系的,空间群 I2/c (No. 15),晶格参数 a = 8.8742 Å、b = 7.609 Å、c = 9.3748 Å 和β = 91.27°。[6]它的晶体结构和溴酸钡同构。[7]
参考资料
- ^ 1.0 1.1 1.2 1.3 Dale L. Perry, Sidney L. Phillips: Handbook of Inorganic Compounds. CRC Press, 1995, ISBN 978-0-8493-8671-8, S. 387 (溴酸锶,第387页,载于Google图书).
- ^ Oliver Sacks. Uncle Tungsten: Memories of a Chemical Boyhood First Vintage Books. 2002: 230.
- ^ Strontium Bromate. American Elements. [25 July 2013]. (原始内容存档于2013-07-22).
- ^ R. Abegg, F. Auerbach: Handbuch der anorganischen Chemie. Verlag S. Hirzel, Bd. 2, 1908. S. 223 (Volltext).
- ^ William F. Linke: The Solubility of the Strontium Halates. In: J. Am. Chem. Soc. 1953, 75(23), p. 5797-5800. doi:10.1021/ja01119a009.
- ^ D. L. Sastry, M. V. Rajasekharan, K. V. S. Rama Rao: An X-ray study of strontium bromate monohydrate. In: Zeitschrift für Kristallographie 1980, 152(3-4), S. 333–334. doi:10.1524/zkri.1980.152.3-4.333
- ^ L. K. Templeton, D. H. Templeton: Structure of barium bromate monohydrate. In: Acta Cryst. 1989, C45, S. 672–673. doi:10.1107/S0108270188013071.
- ^ Richard C. Ropp. Encyclopedia of the Alkaline Earth Compounds. Newnes. 2012: 94. ISBN 0-444-59553-8.
- ^ C. Rammelsberg: Ueber die Bromsäure und deren Salze. In: Pogg. Ann. 1841, 52, S. 79–97 (Volltext (页面存档备份,存于互联网档案馆)).
| |||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.