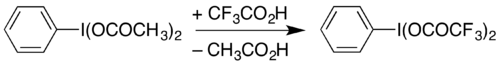二(三氟乙酸)碘苯
| 二(三氟乙酸)碘苯 | |
|---|---|

| |

| |
| IUPAC名 Phenyl-λ3-iodanediyl bis(trifluoroacetate) | |
| 别名 | 双(三氟乙酸)碘苯 二(三氟乙酰氧基)碘苯 PIFA |
| 识别 | |
| CAS号 | 2712-78-9 |
| PubChem | 102317 |
| ChemSpider | 92428 |
| SMILES |
|
| InChI |
|
| InChIKey | PEZNEXFPRSOYPL-UHFFFAOYAA |
| 性质 | |
| 化学式 | C10H5F6IO4 |
| 摩尔质量 | 430.04 g·mol−1 |
| 危险性 | |
GHS危险性符号
| |
| GHS提示词 | Warning |
| H-术语 | H315, H319, H335 |
| P-术语 | P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
二(三氟乙酸)碘苯, 化学式 C
6H
5I(OCOCF
3)
2,是一种超价碘化合物,用作有机化学试剂。在酸性条件下,二(三氟乙酸)碘苯可以引起霍夫曼降解反应。[1]
制备
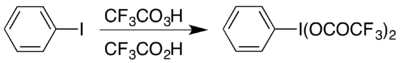
[编辑]所有的芳基超价碘化合物都是从碘苯开始合成的。二(三氟乙酸)碘苯可以通过碘苯和三氟过氧乙酸与三氟乙酸的混合物反应而成。这个方法类似二(乙酸)碘苯的制备方法。[1]
它也可以由二(乙酸)碘苯和三氟乙酸加热反应而成:[2]
用处
[编辑]二(三氟乙酸)碘苯可以把腙转化成重氮化合物,可用于巴顿–凯洛格反应。它也可以把缩硫醛转化成对应的羰基化合物。
霍夫曼降解反应
[编辑]霍夫曼降解反应是酰胺通过异氰酸酯中间体脱羰成胺的反应。这个反应通常在强碱性环境下发生。[3][4]
使用超价碘化合物可以使该反应在弱酸性条件下发生。[1]《有机合成》中发表的一个例子是由环丁基甲酸合成的环丁基甲酰胺转化成环丁胺的反应。[2]反应产生的伯胺以三氟乙酸盐的形式存在,可以通过将其转化成盐酸盐来纯化。[1][2]
参考资料
[编辑]- ^ 1.0 1.1 1.2 1.3 Aubé, Jeffrey; Fehl, Charlie; Liu, Ruzhang; McLeod, Michael C.; Motiwala, Hashim F. 6.15 Hofmann, Curtius, Schmidt, Lossen, and Related Reactions. Heteroatom Manipulations. Comprehensive Organic Synthesis II 6. 1993: 598–635. ISBN 9780080977430. doi:10.1016/B978-0-08-097742-3.00623-6.
- ^ 2.0 2.1 2.2 (1988) "Hofmann Rearrangement Under Mildly Acidic Conditions Using [I,I-Bis(Trifluoroacetoxy)Iodobenzene: Cyclobutylamine Hydrochloride from Cyclobutanecarboxamide]". Org. Synth. 66; Coll. Vol. 8: 132.
- ^ Wallis, Everett S.; Lane, John F. The Hofmann Reaction. Organic Reactions. 1946, 3 (7): 267–306. doi:10.1002/0471264180.or003.07.
- ^ Surrey, Alexander R. Hofmann Reaction. Name Reactions in Organic Chemistry 2nd. Academic Press. 1961: 134–136 [2021-12-29]. ISBN 9781483258683. (原始内容存档于2021-12-29).
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.