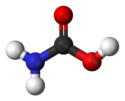
Karbaminska kiselina
| |||
| Nazivi | |||
|---|---|---|---|
| IUPAC naziv
Carbamic acid
| |||
| Identifikacija | |||
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| KEGG[1] | |||
| MeSH | Carbamic+acid | ||
| |||
| Svojstva | |||
| CH3NO2 | |||
| Molarna masa | 61,040 g/mol | ||
| Srodna jedinjenja | |||
Srodna jedinjenja
|
Ditiokarbamat Ugljena kiselina Ureja Etil karbamat | ||
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25 °C [77 °F], 100 kPa). | |||
| Reference infokutije | |||
Karbaminska kiselina je jedinjenje koje je nestabilno pod normalnim uslovima. Ona je tehnički najjednostavnija aminokiselina, mada njena nestabilnost (i jedinstvena priroda karboksil-azotne veze) omogućavaju glicinu do poprimi taj status. Na osnovu njenog imena se formiraju imena većih analoga.[4] Sama karbaminska kiselina nije bila sintetisana.[5]
Radical se zove karbamoil. Karbamoiltransferaze su transferazni enzimi klasifikovan pod EC brojen 2.1.3.
-
karbaminska kiselinska grupa
-
karbamatna grupa
-
karbamoilna grupa
Karbaminske kiseline su intermedijari u razlaganju karbamatnih zaštitnih grupa; hidroliza estarske veze proizvodi karbaminsku kiselinu, iz koje se izdvaja ugljen dioksid, čime nastaje nezaštićeni amin.
Reference
[уреди | уреди извор]- ^ Joanne Wixon; Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast. 17 (1): 48—55. doi:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
- ^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
- ^ Thomas L. Lemke. (2003). Review of organic functional groups : introduction to medicinal organic chemistry. Philadelphia, Pa.: Lippincott Williams & Wilkins. стр. 63. ISBN 978-0-7817-4381-5.
- ^ R.K. Khanna, M.H. Moore. (1998). „A 55”. Carbamic acid: molecular structure and IR spectra (pii: S1386-1425(98)00228-5) (PDF). Greenbelt, MD.: Elsevier. стр. 961—967.
Literatura
[уреди | уреди извор]- Thomas L. Lemke. (2003). Review of organic functional groups : introduction to medicinal organic chemistry. Philadelphia, Pa.: Lippincott Williams & Wilkins. стр. 63. ISBN 978-0-7817-4381-5.
- R.K. Khanna, M.H. Moore. (1998). „A 55”. Carbamic acid: molecular structure and IR spectra (pii: S1386-1425(98)00228-5) (PDF). Greenbelt, MD.: Elsevier. стр. 961—967.
Spoljašnje veze
[уреди | уреди извор]Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.