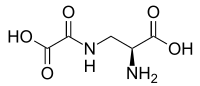
Oksalildiaminopropionska kiselina
| |
| Nazivi | |
|---|---|
| Sistemski IUPAC naziv
(2S)-2-Amino-3-(oksaloamino)propanoinska kiselina | |
Drugi nazivi
| |
| Identifikacija | |
3D model (Jmol)
|
|
| 3DMet | B00693 |
| Abrevijacija |
|
| ChEBI | |
| ChemSpider | |
| KEGG[2] | |
| MeSH | oxalyldiaminopropionic+acid |
| UNII |
|
| |
| Svojstva | |
| C5H8N2O5 | |
| Molarna masa | 176,13 g·mol−1 |
| Srodna jedinjenja | |
Srodna jedinjenja
|
Beta-Metilamino-L-alanin |
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25 °C [77 °F], 100 kPa). | |
| Reference infokutije | |
Oksalildiaminopropionska kiselina (ODAP) je strukturni analog neurotransmitera glutamata koji je prisutan u biljci graška Lathyrus sativus. ODAP je neurotoksin odgovoran za sindrom degeneracije motornih neurona latirizam.[1]
Izvori
[уреди | уреди извор]ODAP je prisutna u semenu mahunarki L. sativus, biljke graška, u rasponu od .5% w/w.[5] L. sativus se može naći u oblastima Južne, Centralne i Istočne Evrope, Mediteranskog basena, Iraka i Avganistana, kao i oblasti Azije i Afrike.[6]
Osobine
[уреди | уреди извор]Oksalildiaminopropionska kiselina je organsko jedinjenje, koje sadrži 5 atoma ugljenika i ima molekulsku masu od 176,127 Da.
| Osobina | Vrednost |
|---|---|
| Broj akceptora vodonika | 6 |
| Broj donora vodonika | 4 |
| Broj rotacionih veza | 4 |
| Particioni koeficijent[7] (ALogP) | -4,5 |
| Rastvorljivost[8] (logS, log(mol/L)) | -1,0 |
| Polarna površina[9] (PSA, Å2) | 129,7 |
Reference
[уреди | уреди извор]- ^ а б Woldeamanuel, Yohannes W.; Hassan, Anhar; Zenebe, Guta (2011-11-12). „Neurolathyrism: two Ethiopian case reports and review of the literature”. Journal of Neurology (на језику: енглески). 259 (7): 1263—1268. ISSN 0340-5354. PMID 22081101. S2CID 27543906. doi:10.1007/s00415-011-6306-4.
- ^ Joanne Wixon; Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast. 17 (1): 48—55. doi:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
- ^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
- ^ Rao, S; Adiga, P; Sarma, P (март 1964). „The Isolation and Characterization of β-N-Oxalyl-L-α,β-Diaminopropionic Acid: A Neurotoxin from the Seeds of Lathyrus sativus”. Biochemistry. 3 (3): 432—436. PMID 14155110. doi:10.1021/bi00891a022.
- ^ Heuzé V., Tran G., Hassoun P., Lessire M., Lebas F., 2016. Grass pea (Lathyrus sativus). Feedipedia, a programme by INRA, CIRAD, AFZ and FAO. https://www.feedipedia.org/node/285 Last updated on April 19, 2016, 15:36
- ^ Ghose, A.K.; Viswanadhan V.N. & Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A. 102: 3762—3772. doi:10.1021/jp980230o.
- ^ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE (2001). „Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”. Chem Inf. Comput. Sci. 41: 1488—1493. PMID 11749573. doi:10.1021/ci000392t.
- ^ Ertl P.; Rohde B.; Selzer P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”. J. Med. Chem. 43: 3714—3717. PMID 11020286. doi:10.1021/jm000942e.
Literatura
[уреди | уреди извор]- Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (I изд.). Oxford University Press. ISBN 978-0-19-850346-0.
- Smith, Michael B.; March, Jerry (2007). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th изд.). New York: Wiley-Interscience. ISBN 0-471-72091-7.
- Katritzky A.R.; Pozharskii A.F. (2000). Handbook of Heterocyclic Chemistry (Second изд.). Academic Press. ISBN 0080429882.
Spoljašnje veze
[уреди | уреди извор]| AMPAR |
|
|---|---|
| KAR |
|
| NMDAR |
|
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
