Adenosinum triphosphoricum
| |

| |
| Cognitores | |
|---|---|
| DrugBank | DB00171 |
| PubChem | 5957 |
| Natura chemica | |
| Formula summarum | C 10H 16N 5O 13P 3 |
| Massa molaris | 507.18 g/mol |
| Potentia acidi | pKa1 = 0.9 pKa2 = 1.5 pKa3 = 2.3 pKa4 = 7.7 |
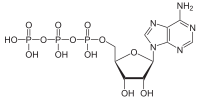
Acidum adenosinum triphosphoricum (abbreviatura: ATP) est nucleotidum et energiae fons laborum corporis. ATP photosynthesi et phosphorylatione oxidativa oritur. Molecula ex grege adenini, saccharo ribosi et tribus gregibus phosphati constituta est. Separatione ex ATP gregis phosphati γ energiam liberat, ut adenosinum diphosphoricum (ADP) restet.[1]
Forma iontibus onerata (sal acidi adenosini triphosphorici) adenosinum triphosphatum nominatur.
Contextus
[recensere | fontem recensere]Omnes organismi functiones energiam requirunt, praecipue musculorum actio magnam copiam energiae consumunt. Energiae corporalis fons principalis est ATP. Verbi gratia, cor humanum die circiter 6 000g ATP consumit.
Historia
[recensere | fontem recensere]Anno 1929 biochemicus Germanicus Carolus Lohmann primus ATP descripsit[2]. Anno 1935 Russicus Vladimirus Engelhart contractiones musculares ex ATP pendere cognovit. Anno 1937 Danicus Hermanus Kalckar intellegit, ut synthesis cum ATPasi conexa sit.
Natura chemica
[recensere | fontem recensere]Structura
[recensere | fontem recensere]Formula summarum sua est C
10H
16N
5O
13P
3. Molecula ex grege adenini, saccharo ribosi et tribus gregibus phosphati constituta est. Adenini anulo duplici in positione nona, quo unus atomus nitrogenii est, saccharum ribosi cum atomo carbonii affixum est. Opposita tres greges phophati iacent.
-
β-D-ribosum
Adeninum
[recensere | fontem recensere]Adeninum est purinum compositum chemicum heterocyclicum et basis nucleici acidi. Heterocyclus ipse ex atomis et carbonii et nitrogenii, "N", compositus, coniunctio duorum anulorum, imidazoli (5 atomis) enim et pyrimidini (6 atomis), est. In organismi metabolismo adeninum ex riboso phosphato (itinere pentosorum phosphatorum) generatur.
Acidum phosphoricum
[recensere | fontem recensere]Acidum phosphoricum est acidum cum formula summarum H
3PO
4. Basis eius coniugata est ion dihydrogenium-phosphatum cum formula summarum H
2PO−
4, cuius basis coniugata hydrogenium phosphatum formula eius summarum est HPO2−
4, denique cuius basis phosphatum nominatur, cum formula summarum PO3−
4. Valores potentiarum acidorum sunt 1.9, 6.7, atque 12.4.
Notae
[recensere | fontem recensere]- ↑ Knowles, J. R. (1980). "Enzyme-catalyzed phosphoryl transfer reactions". Annu. Rev. Biochem. 49: 877–919
- ↑ Situs praemii Nobelii de ATP. (Anglice)
Plura legere si cupis
[recensere | fontem recensere]- R W Hanson, 'The role of ATP in metabolism', Biochemical education, 1989ː 86-92
- Know L. (2018). Mitochondria and the future of medicine. Chelsea Green Publishing, ISBN 978-1-60358-767-9
Nexus interni
Nexus externi
[recensere | fontem recensere]- fons nominis ext.
- nobelprize.org de historia ATP.
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.





