5,10-Methylenetetrahydrofolate

| |
| Names | |
|---|---|
| IUPAC name
N-[4-(3-amino-1-oxo-1,4,5,6,6a,7-hexahydroimidazo[1,5-f]pteridin-8(9H)-yl)benzoyl]-L-glutamic acid
| |
| Other names
5,10-CH2-THF,
MTHF | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | 5,10-methylenetetrahydrofolate |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H23N7O6 | |
| Molar mass | 457.44 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
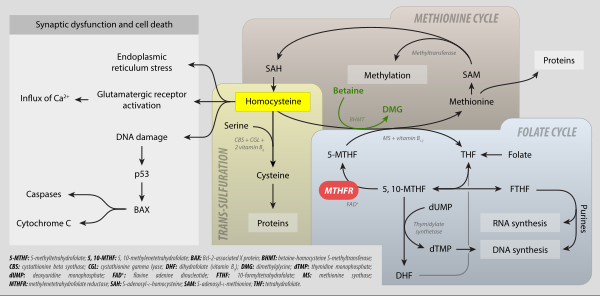
5,10-Methylenetetrahydrofolate (N5,N10-Methylenetetrahydrofolate; 5,10-CH2-THF) is cofactor in several biochemical reactions. It exists in nature as the diastereoisomer [6R]-5,10-methylene-THF.
As an intermediate in one-carbon metabolism, 5,10-CH2-THF converts to 5-methyltetrahydrofolate, 5-formyltetrahydrofolate, and methenyltetrahydrofolate. It is substrate for the enzyme methylenetetrahydrofolate reductase (MTHFR)[1][2] It is mainly produced by the reaction of tetrahydrofolate with serine, catalyzed by the enzyme serine hydroxymethyltransferase.
Selected functions
Formaldehyde equivalent
Methylenetetrahydrofolate is a source of the equivalent of formaldehyde or CH22+ in biosyntheses.
Methylenetetrahydrofolate is also an intermediate in the detoxification of formaldehyde.[3]
Pyrimidine biosynthesis
It is the one-carbon donor for thymidylate synthase, for methylation of 2-deoxy-uridine-5-monophosphate (dUMP) to 2-deoxy-thymidine-5-monophosphate (dTMP). The coenzyme is necessary for the biosynthesis of thymidine and is the C1-donor in the reactions catalyzed by TS and thymidylate synthase (FAD).
Biomodulator
[6R]-5,10-methylene-THF is a biomodulator that has proven to enhance the desired cytotoxic antitumor effect of Fluorouracil (5-FU) and can bypass the metabolic pathway required by other folates (such as leucovorin) to achieve necessary activation.[4] The active metabolite is being evaluated in clinical trials for patients with colorectal cancer in combination with 5-FU.
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
