CUMYL-4CN-BINACA
Chemical compound
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
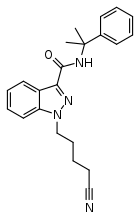
| Formula | C22H24N4O |
| Molar mass | 360.461 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
CUMYL-4CN-BINACA (also known as CUMYL-CYBINACA or SGT-78) is an indazole-3-carboxamide based synthetic cannabinoid that has been sold online as a designer drug.[3][4][5][6][7][8] It is a potent agonist for cannabinoid receptors CB1 and CB2, with in vitro EC50 values of 0.58 nM and 6.12 nM, respectively.[9] In mice, CUMYL-4CN-BINACA produces hypothermic and pro-convulsant effects via the CB1 receptor,[9] and anecdotal reports suggest it has an active dose of around 0.1 mg in humans.[10]
CUMYL-4CN-BINACA is metabolized to produce cyanide, raising concerns about liver toxicity.[8] There is one reported case of hyperthermia, rhabdomyolysis, and kidney failure associated with its use.[11]
- 4CN-AMB-BUTINACA
- 5F-CUMYL-PINACA
- 5F-SDB-006
- ADAMANTYL-THPINACA
- CUMYL-PICA
- CUMYL-PINACA
- CUMYL-THPINACA
- SDB-006
- NNE1
- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ "Substance Details CUMYL-4CN-BINACA". Retrieved 2024-01-22.
- ^ "4-cyano CUMYL-BUTINACA". Cayman Chemical.
- ^ Yeter O (November 2017). "Identification of the Synthetic Cannabinoid 1-(4-cyanobutyl)-N-(2-phenylpropan-2-yl)-1H-indazole-3-carboxamide (CUMYL-4CN-BINACA) in Plant Material and Quantification in Post-Mortem Blood Samples". Journal of Analytical Toxicology. 41 (9): 720–728. doi:10.1002/dta.2248. PMID 28977413.
- ^ Staeheli SN, Poetzsch M, Veloso VP, Bovens M, Bissig C, Steuer AE, Kraemer T (January 2018). "In vitro metabolism of the synthetic cannabinoids CUMYL-PINACA, 5F-CUMYL-PINACA, CUMYL-4CN-BINACA, 5F-CUMYL-P7AICA and CUMYL-4CN-B7AICA". Drug Testing and Analysis. 10 (1): 148–157. doi:10.1002/dta.2298. PMID 28885775.
- ^ Bovens M, Bissig C, Staeheli SN, Poetzsch M, Pfeiffer B, Kraemer T (December 2017). "Structural characterization of the new synthetic cannabinoids CUMYL-PINACA, 5F-CUMYL-PINACA, CUMYL-4CN-BINACA, 5F-CUMYL-P7AICA and CUMYL-4CN-B7AICA". Forensic Science International. 281: 98–105. doi:10.1016/j.forsciint.2017.10.020. PMID 29125990.
- ^ Arıkan Ölmez N, Kapucu H, Çallı Altun N, Eren B (January 2018). "Identification of the synthetic cannabinoid N-(2-phenyl-propan-2-yl)-1-(4-cyanobutyl)-1H-indazole-3-carboxamide (CUMYL-4CN-BINACA) in a herbal mixture product". Forensic Toxicology. 36 (1): 192–199. doi:10.1007/s11419-017-0372-y. ISSN 1860-8965. S2CID 28058750.
- ^ a b Åstrand A, Vikingsson S, Lindstedt D, Thelander G, Gréen H, Kronstrand R, Wohlfarth A (March 2018). "Metabolism study for CUMYL-4CN-BINACA in human hepatocytes and authentic urine specimens: Free cyanide is formed during the main metabolic pathway". Drug Testing and Analysis. 10 (8): 1270–1279. doi:10.1002/dta.2373. PMID 29577658.
- ^ a b Kevin RC, Anderson L, McGregor IS, Boyd R, Manning JJ, Glass M, et al. (2019). "CUMYL-4CN-BINACA Is an Efficacious and Potent Pro-Convulsant Synthetic Cannabinoid Receptor Agonist". Frontiers in Pharmacology. 10: 595. doi:10.3389/fphar.2019.00595. PMC 6549035. PMID 31191320.
- ^ "EMCDDA–Europol Joint Report on a new psychoactive substance: 1-(4-cyanobutyl)-N-(2-phenylpropan-2-yl)indazole-3-carboxamide (CUMYL-4CN-BINACA)". www.emcdda.europa.eu.
- ^ El Zahran T, Gerona R, Morgan BW, Pomerleau AC (June 2019). "A novel synthetic cannabinoid (Cumyl-4-cyano-BINACA) resulting in hyperthermia, rhabdomyolysis, and renal failure in a 29-year-old patient: it's not meningitis". Clinical Toxicology. 57 (6): 421–422. doi:10.1080/15563650.2018.1534241. PMID 30442067. S2CID 53566116.
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (modulators) |
| ||||||||||||||||||||||||||||||
| Enzyme (modulators) |
| ||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
Cover photo is available under {{::mainImage.info.license.name || 'Unknown'}} license.
Cover photo is available under {{::mainImage.info.license.name || 'Unknown'}} license.
Credit:
(see original file).
