1,2-双(二苯基膦)乙烷
| 1,2-双(二苯基膦)乙烷 | |
|---|---|

| |
| |
| IUPAC名 乙烷-1,2-双(二苯基膦) | |
| 别名 | Diphos Dppe |
| 识别 | |
| CAS号 | 1663-45-2 |
| ChemSpider | 66873 |
| SMILES |
|
| InChI |
|
| InChIKey | QFMZQPDHXULLKC-UHFFFAOYAX |
| ChEBI | 30669 |
| 性质 | |
| 化学式 | C26H24P2 |
| 摩尔质量 | 398.42 g·mol⁻¹ |
| 熔点 | 140-142 °C |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
1,2-双(二苯基膦)乙烷(英語:1,2-Bis(diphenylphosphino)ethane,缩写:dppe)是一种属于二膦的有机化合物,在有机金属化学和配位化学中常用作双齿配体。其配合物多为螯合物,但偶尔也能形成单齿(如W(CO)5(dppe))或桥联的配合物。[1]
制备
[编辑]制备dppe可通过NaPPh2的烷基化进行,而制备NaPPh2通常可从三苯基膦(P(C6H5)3)如下制备:[2][3]
- P(C6H5)3 + 2 Na → NaP(C6H5)2 + NaC6H5
NaP(C6H5)2 得到的有机膦钠化合物在空气中易于氧化,可继续与1,2-二氯乙烷(ClCH2CH2Cl)反应得到dppe:
- 2 NaP(C6H5)2 + ClCH2CH2Cl → (C6H5)2PCH2CH2P(C6H5)2 + 2 NaCl
反应
[编辑]还原反应
[编辑]据报道,可使用锂还原dppe得到PhHP(CH2)2PHPh。[4]
与水dppe发生水解反应:
氧化反应
[编辑]使用常规氧化剂〔如过氧化氢(H2O2)或溴素(Br2)等〕处理dppe,通常得到dppeO的收率很低(如13%收率),这是由于反应的非选择性而最后得到:原料、单氧化物和双氧化物的混合产物。[5] 使用PhCH2Br与dppe发生选择性的单氧化反应则可较高收率的得到dppeO。
- Ph2P(CH2)2PPh2 + PhCH2Br → Ph2P(CH2)2PPh2(CH2Ph)+Br-
继而进行纯化得到单-鏻盐[需要解释],并进行碱催化的水解反应。
- Ph2P(CH2)2PPh2(CH2Ph)+Br- + NaOH + H2O → Ph2P(CH2)2P(O)Ph2
配位化学
[编辑]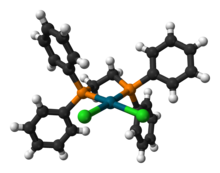
dppe与相关二膦配体形成的金属配合物,在各类化学反应中大多用于均相催化剂。常用的两种dppe配位络合物为:Pd(dppe)2和Ir(dppe)2。Pd(dppe)2可通过Pd(II)和NaBH4发生还原反应得到,但原位从乙酸钯(II)制备是最简便的方法。[5]
参见
[编辑]参考文献
[编辑]- ^ Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry: A Comprehensive Text, 4th ed.; Wiley-Interscience Publications: New York, NY, 1980; p.246. ISBN 0-471-02775-8
- ^ W. Hewertson and H. R. Watson. 283. The preparation of di- and tri-tertiary phosphines. J. Chem. Soc. 1962: 1490–1494. doi:10.1039/JR9620001490.
- ^ Girolami, G.; Rauchfuss, T.; Angelici, R. Synthesis and Technique in Inorganic Chemistry, 3rd ed.; University Science Books: Sausalito, CA, 1999; pp. 85-92. ISBN 0-935702-48-2
- ^ Dogan, J.; Schulte, J.B.; Swiegers, G.F.; Wild, S.B. Mechanism of Phosphorus-Carbon Bond Cleavage by Lithium in Tertiary Phosphines. An Optimized Synthesis of 1, 2-Bis (phenylphosphino) ethane. J. Org. Chem. 2000, 65 (4): 951–957. doi:10.1021/jo9907336.
- ^ 5.0 5.1 Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons, Ltd
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
