For faster navigation, this Iframe is preloading the Wikiwand page for
舒尼替尼 .
舒尼替尼 臨床資料 商品名 索坦 AHFS /Drugs.com Monograph MedlinePlus a607052 核准狀況
懷孕分級 给药途径 口服 ATC碼 法律規範狀態 法律規範
藥物動力學 數據生物利用度 不受食物影响 血漿蛋白結合率 95% 药物代谢 肝脏 (CYP3A4 参与代谢)生物半衰期 40至60小时(舒尼替尼) 排泄途徑 61% 粪便;16% 肾脏 识别信息
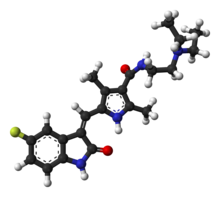
N -(2-diethylaminoethyl)-5-[(Z )-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H -pyrrole-3-carboxamide
PubChem CID DrugBank ChemSpider UNII KEGG ChEBI ChEMBL CompTox Dashboard (EPA ) 化学信息 化学式 C 22 H 27 F N 4 O 2 摩尔质量 398.474 g/mol 3D模型(JSmol
CCN(CC)CCNC(=O)c1c(c([nH]c1C)/C=C\2/c3cc(ccc3NC2=O)F)C
InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12-
Y Key:WINHZLLDWRZWRT-ATVHPVEESA-N
Y
舒尼替尼 (由辉瑞 研发,商标名索坦 )是一种口服的小分子多靶点受体酪氨酸激酶 抑制剂,2006年1月26日被FDA 批准用于治疗对标准疗法没有响应或不能耐受的胃肠道基质肿瘤 和转移性肾细胞癌。舒尼替尼是第一种被批准用于同时治疗两种类型癌症 的药物。[1]
舒尼替尼通过靶向多个受体酪氨酸激酶来抑制细胞信号。
这些受体 包括:血小板衍化生长因子R链(PDGF-R)和血管内皮生长因子 受体(VEGFRs),它们在肿瘤的血管生成 和细胞增殖中扮演重要角色。这些同时进行的抑制促使肿瘤血管化减少并使癌症发生细胞凋亡 ,最终使肿瘤萎缩。
舒尼替尼同样抑制KIT基因(CD117),[2] [3] 伊马替尼 抗药,或是服药耐受性降低。[4] [5]
另外,舒尼替尼抑制其它受体酪氨酸激酶[6]
由于舒尼替尼针对许多不同受体,它也带来很多副作用,例如手足病、口腔炎 和其它皮肤性中毒。
癌症免疫疗法 (CI )单克隆抗体药物
其它实体瘤
淋巴 :CD20
替伊莫单抗 奥滨尤妥珠单抗 奥法木单抗 利妥昔單抗 托西莫单抗 CD30
CD52
免疫检查点抑制剂
酪氨酸激酶抑制剂
RET 抑制剂:恩曲替尼(也作用于ALK、ROS1、NTRK), 拉罗替尼(也作用于NTRK), 英菲格拉替尼, 培米替尼(也作用于FGFR), 普拉替尼, 塞尔帕替尼(也作用于VEGFR和EGFR), 凡德他尼
c-MET 抑制剂:卡博替尼
非受体酪氨酸激酶
其它
hedgehog抑制剂
Glasdegib
Sonidegib
维斯莫吉布 CDK抑制剂
玻玛西林
帕博西尼
瑞博西尼
Trilaciclib 卡博替尼
卡马替尼
恩曲替尼
埃达非替尼
吉特替尼
拉罗替尼
乐伐替尼
马赛替尼 米哚妥林
尼達尼布 帕唑帕尼
派米加替尼
培西达替尼 奎扎替尼 瑞戈非尼
利普替尼
索托拉西布
舒尼替尼 特泊替尼
凡德他尼
维奈托克
趋化因子受体 集落刺激因子 (CSF)
促紅細胞生成素受體
激動劑: ARA-290Asialo erythropoietin
Carbamylated erythropoietin
CNTO-530
Darbepoetin alfa
Epoetin alfa
Epoetin beta
Epoetin delta
Epoetin epsilon
Epoetin gamma
Epoetin kappa
Epoetin omega
Epoetin theta
紅血球生成素ζ 紅血球生成素 (EPO)Erythropoietin-Fc
Methoxy polyethylene glycol-epoetin beta (CERA/Mircera)
Peginesatide
Pegol sihematide (EPO-018B) G-CSF (CSF3)
激動劑: FilgrastimGranulocyte colony-stimulating factor
Lenograstim
Leridistim
Lipegfilgrastim
Nartograstim
Pegfilgrastim
Pegnartograstim GM-CSF (CSF2)
激動劑: Mavrilimumab纳米鲁单抗 Otilimab M-CSF (CSF1)
激動劑: CilmostimInterleukin-34
Lanimostim
Macrophage colony-stimulating factor
Mirimostim SCF (c-Kit) 血小板生成素受體
激動劑: EltrombopagPegacaristim
Promegapoietin
Romiplostim
促血小板生成素 (THPO, MGDF)
細胞因子受體
IFNAR (α/β, I)
激動劑: AlbinterferonInterferon alpha (interferon alfa, IFN-α)
Interferon alfa (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21)
Interferon alfa 2a
Interferon alfa 2b
Interferon alfa n1
Interferon alfacon-1
Interferon alpha-n3
Interferon beta (IFN-β) (IFNB1, IFNB3)
Interferon beta 1a
Interferon beta 1b
Interferon kappa (IFN-ε/κ/τ/ζ, IFNK)
Interferon omega (IFN-ω, IFNW1)
Peginterferon alfa-2a
Peginterferon alfa-2b 抗體: AnifrolumabFaralimomab
MEDI-545
Rontalizumab
Sifalimumab IFNGR (γ, II)
抗體: γ-干扰素 (IFN-γ)Interferon gamma 1b 抗體: EmapalumabFontolizumab IFNLR (λ, III)
白介素受體 TGFβ型受體 TNF受體超家族 其它
JAK激酶 (抑製劑
JAK激酶1
Baricitinib
Filgotinib
Momelotinib
Oclacitinib
Ruxolitinib
Tofacitinib (tasocitinib, CP-690550)
Upadacitinib JAK激酶2
AG-490
Atiprimod
AZD-1480
Baricitinib
CHZ868
葫蘆素 (埃利黴素B CYT387
Lestaurtinib
NSC-7908
NSC-33994
Pacritinib
Ruxolitinib
SD-1008
Tofacitinib (tasocitinib, CP-690550) JAK激酶3
AG-490
Cercosporamide
Decernotinib (VX-509)
TCS-21311
Tofacitinib (tasocitinib, CP-690550)
WHI-P 154
ZM-39923
ZM-449829
其它
其他細胞因子: Cardiotrophin 1 (CT-1)FMS-like tyrosine kinase 3 ligand (FLT3L)
白血病抑制因子 (LIF)抑癌蛋白M (OSM)Thymic stromal lymphopoietin (TSLP) 其他細胞因子受體調節劑: EmfilerminLestaurtinib
Midostaurin
Quizartinib
索拉非尼 舒尼替尼
{{bottomLinkPreText}}
{{bottomLinkText}}
This page is based on a Wikipedia article written by
contributors (read /edit ).CC BY-SA 4.0 license; additional terms may apply.
{{current.index+1}} of {{items.length}}
Thanks for reporting this video!
This browser is not supported by Wikiwand :(
An extension you use may be preventing Wikiwand articles from loading properly.HTTPS Everywhere or you're unable to access any article on Wikiwand, please consider switching to HTTPS (https ://www.wikiwand.com).
An extension you use may be preventing Wikiwand articles from loading properly.Ad-Blocker , it might have mistakenly blocked our content.
You will need to temporarily disable your Ad-blocker to view this page.
✕
This article was just edited, click to reload
Please click Add in the dialog above
Please click Allow in the top-left corner, Install Now in the dialog
Please click Open in the download dialog, Install
Please click the "Downloads" icon in the Safari toolbar, open the first download in the list, Install
{{::$root.activation.text}}
Follow Us
Don't forget to rate us
Oh no, there's been an error
Please help us solve this error by emailing us at
support@wikiwand.com
Let us know what you've done that caused this error, what browser you're using, and whether you have any special extensions/add-ons installed.
Thank you!