丙二醛
| 丙二醛 | |||
|---|---|---|---|
| |||
| |||
| IUPAC名 propanedial | |||
| 英文名 | Malondialdehyde | ||
| 识别 | |||
| 缩写 | MDA | ||
| CAS号 | 542-78-9 | ||
| PubChem | 10964 | ||
| ChemSpider | 10499 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | WSMYVTOQOOLQHP-UHFFFAOYAU | ||
| KEGG | C19440 | ||
| 性质 | |||
| 化学式 | C3H4O2 | ||
| 摩尔质量 | 72.06 g·mol−1 | ||
| 外观 | 针状固体[1] | ||
| 密度 | 0.991 g/mL | ||
| 熔点 | 72 °C(345 K) | ||
| 沸点 | 108 °C(381 K) | ||
| 危险性 | |||
| PEL | none[1] | ||
| 相关物质 | |||
| 相关烯醛 | 羟基丙二醛 4-羟基-2-壬烯醛 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
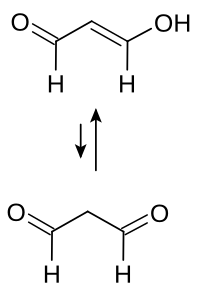
丙二醛,简称MDA,是一种有机化合物,化学式 CH2(CHO)2。它是一种无色液体,反应性极高,通常以烯醇式存在。[2]
结构和制备
[编辑]- CH2(CHO)2 → HOC(H)=CH-CHO
在实验室中,丙二醛可以由对应的缩醛1,1,3,3-四甲氧基丙烷水解而成。与丙二醛不同,这种缩醛是稳定,可商购的。[2]
生物合成和反应性
[编辑]丙二醛可以由脂肪的脂质过氧化而成。[3]它是血栓素A2的合成中的重要产物,其中环加氧酶1或环加氧酶2通过血小板和一系列其他细胞和组织,将花生四烯酸代谢成前列腺素H2。前列腺素H2再被血栓烷合成酶进一步代谢为血栓素A2、12-羟基十七碳三烯酸和丙二醛。[4][5]
活性氧类会降解脂肪,形成丙二醛。[6]这种醛具有高反应性,是许多反应性亲电试剂之一,可在细胞中引起毒性应激并形成称为高级脂肪氧化终产物 (ALE) 的共价蛋白质加合物,类似于高级糖化终产物(AGE)。[7]这种醛的产生可用来测量生物的氧化应激水平。[8][9]
丙二醛会和DNA中的脱氧腺苷和脱氧鸟苷反应,形成DNA加合物,主要会产生突变原M1G。[10]精氨酸的胍基会与丙二醛缩合,得到2-氨基嘧啶。
分析
[编辑]丙二醛和其它硫代巴比妥酸反应性物质 (TBARS) 会与两倍的硫代巴比妥酸缩合,生成可通过分光光度法测定的荧光红色衍生物。[2][11] 1-甲基-2-苯基吲哚是另一种更具选择性的试剂。[2]
危险性和病理学
[编辑]丙二醛具有高反应性,有潜在的致突变性。[12] 它被发现存在于加热的食用油中,例如向日葵油和棕榈油。[13]
根据一项研究,患有圆锥角膜和大疱性角膜病变的患者的角膜中,丙二醛的水平升高了。[14]丙二醛也可以在骨关节炎患者的关节组织切片中找到。[15]
在评估精子中的膜损伤时,也可以考虑丙二醛的水平(脂质过氧化的标志)。这是至关重要的,因为氧化应激通过改变膜流动性、渗透性和损害精子功能来影响精子功能。[16]
参见
[编辑]参考资料
[编辑]- ^ 1.0 1.1 NIOSH Pocket Guide to Chemical Hazards. #0377. NIOSH.
- ^ 2.0 2.1 2.2 2.3 2.4 V. Nair, C. L. O'Neil, P. G. Wang "Malondialdehyde", Encyclopedia of Reagents for Organic Synthesis, 2008, John Wiley & Sons, New York. doi:10.1002/047084289X.rm013.pub2 Article Online Posting Date: March 14, 2008
- ^ Davey MW1, Stals E, Panis B, Keulemans J, Swennen RL. High-throughput determination of malondialdehyde in plant tissues. Analytical Biochemistry. 2005, 347 (2): 201–207. PMID 16289006. doi:10.1016/j.ab.2005.09.041.
- ^ J. Biol. Chem. 248:5673; 1973
- ^ Proc. Natl. Acad. Sci. USA 71:3400; 1974
- ^ Pryor WA, Stanley JP. Letter: A suggested mechanism for the production of malondialdehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J. Org. Chem. 1975, 40 (24): 3615–7. PMID 1185332. doi:10.1021/jo00912a038.
- ^ Farmer EE, Davoine C. Reactive electrophile species. Curr. Opin. Plant Biol. 2007, 10 (4): 380–6. PMID 17646124. doi:10.1016/j.pbi.2007.04.019.
- ^ Moore K, Roberts LJ. Measurement of lipid peroxidation. Free Radic. Res. 1998, 28 (6): 659–71. PMID 9736317. doi:10.3109/10715769809065821.
- ^ Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005, 15 (4): 316–28. PMID 16054557. doi:10.1016/j.numecd.2005.05.003.
- ^ Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 1999, 424 (1–2): 83–95. PMID 10064852. doi:10.1016/S0027-5107(99)00010-X.
- ^ http://www.amdcc.org/shared/showFile.aspx?doctypeid=3&docid=33 互联网档案馆的存檔,存档日期September 14, 2006,.
- ^ Hartman PE, Putative mutagens and carcinogens in foods. IV. Malonaldehyde (malondialdehyde) Environ Mutagen. 1983;5(4):603-7
- ^ Dourerdjou, P.; Koner, B. C. (2008), Effect of Different Cooking Vessels on Heat-Induced Lipid Peroxidation of Different Edible Oils" Journal of Food Biochemistry, 32: 740–751. doi:10.1111/j.1745-4514.2008.00195.x
- ^ Buddi R, Lin B, Atilano SR, Zorapapel NC, Kenney MC, Brown DJ. Evidence of oxidative stress in human corneal diseases. J. Histochem. Cytochem. March 2002, 50 (3): 341–51. PMID 11850437. doi:10.1177/002215540205000306
 .[永久失效連結]
.[永久失效連結]
- ^ Tiku ML, Narla H, Jain M, Yalamanchili P. Glucosamine prevents in vitro collagen degradation in chondrocytes by inhibiting advanced lipoxidation reactions and protein oxidation. Arthritis Research & Therapy. 2007, 9 (4): R76. PMC 2206377
 . PMID 17686167. doi:10.1186/ar2274.
. PMID 17686167. doi:10.1186/ar2274.
- ^ Collodel, G.; Moretti, E.; Micheli, L.; Menchiari, A.; Moltoni, L.; Cerretani, D. Semen characteristics and malondialdehyde levels in men with different reproductive problems. Andrology. March 2015, 3 (2): 280–286. PMID 25331426. S2CID 28027300. doi:10.1111/andr.297 (英语).
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.