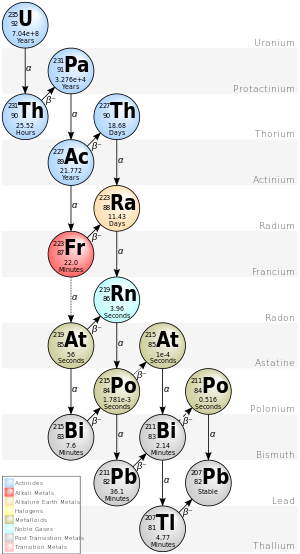
锕衰变链
锕衰变链是原始放射性核素铀-235的衰变链,是自然界中现存的三大衰变链之一。该衰变链的起始核素为铀-235,半衰期长达7.038亿年,衰变产物包括以下几种元素的短寿命放射性同位素:钍、镤、锕、钫、镭、砹、氡、铋、钋、铅和铊,它们都短暂或长期地存在于任何含有铀-235的铀金属、矿藏、化合物或合金中。该衰变链的最终产物为稳定的铅-207。
本衰变链中的每个核素的质量数可以下式表示:A = 4n + 3,故本衰变链亦称为4n + 3系列。[1]
该链从铀-235衰变成铅-207所释放的总能量(包括放出中微子损失的能量)为46.4MeV。
| 核素 | 旧元素符号 | 旧称 | 衰变形式 | 半衰期 | 释放能量,MeV[a] | 衰变产物 |
|---|---|---|---|---|---|---|
| 251Cf | α | 900.6年 | 6.176 | 247Cm | ||
| 247Cm | α | 1.56·107年 | 5.353 | 243Pu | ||
| 243Pu | β− | 4.95556小时 | 0.579 | 243Am | ||
| 243Am | α | 7388年 | 5.439 | 239Np | ||
| 239Np | β− | 2.3565天 | 0.723 | 239Pu | ||
| 239Pu | α | 24100年 | 5.244 | 235U | ||
| 235U | AcU | 锕铀(Actin Uranium) | α | 7.04·108年 | 4.678 | 231Th |
| 231Th | UY | 铀Y(Uranium Y) | β− | 25.52小时 | 0.391 | 231Pa |
| 231Pa | Pa | 镤 / 原锕(Protactinium) | α | 32760年 | 5.150 | 227Ac |
| 227Ac | Ac | 锕(Actinium) | β− 98.62% α 1.38% |
21.772年 | 0.045 5.042 |
227Th 223Fr |
| 227Th | RdAc | 射锕(Radioactinium) | α | 18.68天 | 6.147 | 223Ra |
| 223Fr | AcK | 锕K(Actinium K) | β− 99.994% α 0.006% |
22.00分钟 | 1.149 5.340 |
223Ra 219At |
| 223Ra | AcX | 锕X(Actinium X) | α | 11.43天 | 5.979 | 219Rn |
| 219At | α 97.00% β− 3.00% |
56秒 | 6.275 1.700 |
215Bi 219Rn | ||
| 219Rn | An | 锕射气(Actinon; Actinium Emanation) |
α | 3.96秒 | 6.946 | 215Po |
| 215Bi | β− | 7.6分钟 | 2.250 | 215Po | ||
| 215Po | AcA | 锕A(Actinium A) | α 99.99977% β− 0.00023% |
1.781毫秒 | 7.527 0.715 |
211Pb 215At |
| 215At | α | 0.1毫秒 | 8.178 | 211Bi | ||
| 211Pb | AcB | 锕B(Actinium B) | β− | 36.1分钟 | 1.367 | 211Bi |
| 211Bi | AcC | 锕C(Actinium C) | α 99.724% β− 0.276% |
2.14分钟 | 6.751 0.575 |
207Tl 211Po |
| 211Po | AcC' | 锕C'(Actinium C') | α | 516毫秒 | 7.595 | 207Pb |
| 207Tl | AcC" | 锕C"(Actinium C") | β− | 4.77分钟 | 1.418 | 207Pb |
| 207Pb | AcD | 锕D(Actinium D) | - | 稳定 | - | 无 |
- ^ “释放能量”不等于衰变射线的能量。如果为α衰变,α粒子能量比“释放能量”偏小。如果为β衰变,β射线能量为连续谱,最大不超过该能量。详细衰变数据可参考:Berkeley Laboratory Isotopes Project 整理的相关数据: https://web.archive.org/web/20061205022425/http://ie.lbl.gov/education/isotopes.htm 或者 NNDC, Brookhaven National Laboratory 整理的相关数据:http://www.nndc.bnl.gov/chart/ (页面存档备份,存于互联网档案馆) 。
参见
参考文献
- ^ 叶锡溶 蔡长书. 放射化學(第二版). 台湾台北县: 新文京开发出版股份有限公司. 2008-03-26. ISBN 978-986-150-830-6 (中文(台湾)).
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
