配位键
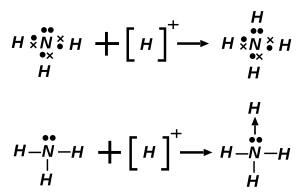
配位键(英语:Coordinate Covalent Bond,又称配位共价键,或简称配键),是一种特殊的共价键。当共价键中共用的电子对是由其中一原子独自供应时,就称配位键。配位键形成后,与一般共价键无异。
相关概念
形成条件
配位键的形成需要两个条件:
- 一是中心原子或离子,它必须有能接受电子对的空轨域;
- 二是配位体,组成y配位体的原子必须能提供配对的孤对电子(L.P)。
当一个路易斯碱供应电子对给路易斯酸而形成化合物时,配位键就形成。
例如:
化合价
在配位化合物中,由电负性小的元素原子向电负性大的元素原子提供孤对电子形成配位键时,每个有一对孤对电子的前者(电负性小的原子)显示+2价,后者显示-2价。反之,由电负性大的元素原子提供孤对电子与电负性小的元素原子之间形成配位键时,两种元素都无价态变化。
常见配位键化合物
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.










