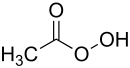
过氧乙酸
| 过氧乙酸 | |||
|---|---|---|---|
| |||
| IUPAC名 ethaneperoxoic acid | |||
| 别名 | 过氧醋酸、过乙酸、过醋酸 | ||
| 识别 | |||
| 缩写 | PAA | ||
| CAS号 | 79-21-0 | ||
| PubChem | 6585 | ||
| ChemSpider | 6336 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | KFSLWBXXFJQRDL-UHFFFAOYAD | ||
| RTECS | SD8750000 | ||
| KEGG | D03467 | ||
| 性质 | |||
| 化学式 | C2H4O3 | ||
| 摩尔质量 | 76.05 g·mol⁻¹ | ||
| 外观 | 无色液体 | ||
| 密度 | 1.13 g/ml (液态) | ||
| 熔点 | 0.1 °C | ||
| 沸点 | 105 °C | ||
| pKa | 8.20 | ||
| 黏度 | 3.280 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
过氧乙酸(俗名过醋酸,英文:Peracetic acid/peroxyacetic acid/PAA)是有机过氧酸家族的成员。透明液体,有典型乙酸气味,是强氧化剂。[1]
历史
[编辑]过醋酸用作消毒剂已有百年历史[2],1902年Freer与Novy于美国化学期刊(American Chemical Journal,ACJ)首次发表过醋酸能有效抑制细菌及细菌孢子活性。1949年美国细菌学协会(American Bacteriological Association)指出有报导比较了灭菌剂23种成分,当中认为过醋酸最有效[3]。
应用
[编辑]现今过醋酸在欧洲大量用于消毒食品工业、酿酒工业、水处理系统及净水系统;在医疗领域,过醋酸用于消毒可重用医疗器械、血液透析设备、医院织物洗涤厂(按德国罗伯特·科赫研究所清单程序)。
过醋酸从1955年即用作消毒剂或灭菌剂,大部分用于处理食品及污水,亦曾用于塑胶隔离用具或消毒医疗物品。它可杀死细菌繁殖体、霉菌、细菌孢子及病毒[4],快速广效;浓度低于百万份之1时,可在5分钟内抑制革兰氏阳性菌、革兰氏阴性菌、霉菌及酵母菌,亦证实可杀死小儿麻痹病毒、轮状病毒、乙型肝炎、HIV病毒。[5][6][7]
化学特性
[编辑]过氧乙酸高浓度时无色、有强烈刺激味、有腐蚀性,高温加热分解,由醋酸及双氧水反应生成,杀菌作用机理为释出自由氧及氢基,最终分解成氧气、水及醋酸。[2]大多市售过醋酸浓5%、10%及15%。
环保优势
[编辑]过醋酸在自然环境中降解为醋酸,再降解为其它物质[8],常用于处理环境河川水。[2]
参考资料
[编辑]- ^ 邢其毅等。. 《基础有机化学》第三版上册. 北京:高等教育出版社. 2005年. ISBN 7-04-016637-2.
- ^ 2.0 2.1 2.2 Dr. Holger Biering. More than 100 years of Peracetic Acid. Journal for Hygiene in Hospitals and Medical Practice.
- ^ I.J. Hutchings u. H. Xezones: Proceedings of the 49th annual meeting of the Society of American Bacteriology, Cincinatti, OH (1949)
- ^ BSG Endoscopy committee working party. Cleaning and disinfection of equipment for gastrointestinal endoscopy. Report of a Working Party of the British Society of Gastroen terology Endoscopy Committee. Gut, 1998; 42:585-93.
- ^ Ossia-Ongagna Y, Sabatier R. Comparison of in vitro activity of six disinfectants on bacteria from contamination in haemodialysis water. J Pharm Belg 1993; 48: 341-5.
- ^ Baldry MGC. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and pancreatic acid. J Appl Bacteriol 1983; 54:417-23.
- ^ Lynam PA, Babb JR, Fraise A. Comparison of the mycobactericidal activity of 2% alkaline glutaraldehyde and Nu-Cidex (0.35% peracetic acid). J Hosp Infect 1995; 30: 237-40.
- ^ ECETOC-Monographie JACC No. 40 »Per- acetic Acid and its Equilibrium Solutions« (2001)
|
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.