蒙脱石
| 蒙脱石 | |
|---|---|
 蒙脱石样品 | |
| 基本资料 | |
| 类别 | 页矽酸盐矿物 粘土矿物 |
| 化学式 | (Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O |
| IMA记号 | Mnt[1] |
| 晶体分类 | 棱柱体 (2/m) (H-M记号相同) |
| 晶体空间群 | C2/m |
| 晶胞 | a = 5.19 Å, b = 9.02 Å, c = 12.4 Å; β = 94°; Z = 2 |
| 性质 | |
| 颜色 | 白色,或浅粉色,蓝,黄,红,绿色 |
| 晶体惯态 | 层状或球状微晶紧密聚集体 |
| 晶系 | 单斜晶系 |
| 解理 | {001}完全 |
| 断口 | 不均匀 |
| 莫氏硬度 | 1–2 |
| 光泽 | 暗淡光泽/土状光泽 |
| 透明性 | 半透明 |
| 比重 | 1.7-2 |
| 光学性质 | 双轴(-) |
| 折射率 | nα = 1.485–1.535 nβ = 1.504–1.550 nγ = 1.505–1.550 |
| 双折射 | δ = 0.020 |
| 2V夹角 | 测量值: 5° to 30° |
| 参考文献 | [2][3][4][5] |
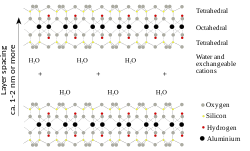
蒙脱石(英语:Montmorillonite)是一种很软的页矽酸盐矿物,属于蒙皂石族的粘土矿物,形成于水中沉降的微晶聚集体。命名取自法国城市蒙莫里永。蒙脱石是一种2:1的黏土,即微观结构上为两层矽氧四面体夹一层铝氧八面体,铝的位置部分被镁同晶取代。蒙脱石片状颗粒平均直径为1微米,厚度0.96纳米。在25,000倍放大的电子显微镜下可见单个黏土颗粒。蒙皂石族的矿物还有皂石。
蒙脱石的化学组成为水合钠钙铝镁矽酸盐氢氧化物,即(Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O。含水量不一定是固定数值,因为蒙脱石单晶间结合并不紧密,水容易渗入,导致黏土显著膨胀。阳离子可被钾,铁等其他离子取代,有色离子的取代则使矿物呈现不同颜色,各地出产的蒙脱石,各种离子的比例不尽相同。它常与绿泥石、白云母、伊利石、焦炭石和高岭石等矿物共存。
发现
形成
蒙脱石可以在洞穴环境中形成和转化。洞穴的自然侵蚀过程中,铝矽酸盐溶解在水中,蒙脱石微晶在漫长的自然作用下形成。高浓度的HCO3-有利于此过程。蒙脱石在干燥环境可转化为坡缕石,或者在pH值小于5的环境下转化为禾乐石-10Å(多水高岭土),再于干燥环境下继续转化为禾乐石-7Å。[6]
应用
石油钻探工业中,蒙脱石用作钻井泥浆的组分,其目的是保持钻头冷却并除去钻出的固体。数十年来,蒙脱石等黏土广泛用于使用工业的裂化催化剂基底,而一些酸基催化剂则使用酸处理的蒙脱石等粘土。 [7]用于改良土壤,在易受干旱影响的地区改善土壤的保水能力。用于土质堤坝的建造,防止水的渗漏。在砂铸件中用作干燥剂,除去空气和其他气体中的水分。
药用
蒙脱石具有很强的吸附性,包括吸附重金属的能力。[8]口服用药称蒙脱石散,是一种常见的治疗急慢性腹泻的药物。或者外用用于预防和治疗接触性皮炎。[9]
参考资料
- ^ Warr, L.N. IMA–CNMNC approved mineral symbols. Mineralogical Magazine. 2021, 85 (3): 291–320. Bibcode:2021MinM...85..291W. S2CID 235729616. doi:10.1180/mgm.2021.43
 .
.
- ^ Mineralienatlas. [2017-03-09]. (原始内容存档于2016-12-25).
- ^ Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C. (编). Montmorillonite. Handbook of Mineralogy (PDF). II (Silica, Silicates). Chantilly, VA, US: Mineralogical Society of America. [December 5, 2011]. ISBN 0962209716. (原始内容 (PDF)存档于2012-02-05).
- ^ 4.0 4.1 Montmorillonite (页面存档备份,存于互联网档案馆). Mindat.org
- ^ Montmorillonite (页面存档备份,存于互联网档案馆). Webmineral
- ^ Hill, Carol; Paolo Forti. Deposition and Stability of asdSilicate Minerals. Cave Minerals of the World Second. National Speleological Society. 1997: 177. ISBN 1-879961-07-5.
- ^ Lloyd, Lawrie. Handbook of Industrial Catalysts. New York: Springer. 2011: 181–182. ISBN 978-0387246826.
- ^ Bhattacharyya, KG; Gupta, SS. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Advances in Colloid and Interface Science. 2008, 140 (2): 114–31. PMID 18319190. doi:10.1016/j.cis.2007.12.008.
- ^ Saary, J; Qureshi, R; Palda, V; Dekoven, J; Pratt, M; Skotnicki-Grant, S; Holness, L. A systematic review of contact dermatitis treatment and prevention. Journal of the American Academy of Dermatology. 2005, 53 (5): 845. PMID 16243136. doi:10.1016/j.jaad.2005.04.075.
参考文献
- Papke, Keith G. Montmorillonite, Bentonite and Fuller’s Earth Deposits in Nevada, Nevada Bureau of Mines Bulletin 76, Mackay School of Mines, University of Nevada-Reno, 1970.
- Mineral Galleries
- Mineral web (页面存档备份,存于互联网档案馆)
Text is available under the CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
